When I started in Psychiatry, if someone said they were going to do a study treating 4041 depressed patients [confirmed by their scores on a rating scale] using a set of sequenced treatment alternatives to relieve depression, I would have had a blank look, because then, depression wasn’t an entity. It was an emotion, an aspect of human experience, a signal from the interior. As a symptom, depression had an almost unending differential diagnosis [including a few who had Depression that did appear to be an ‘entity’]. And if I then read the opening lines of a paper about those sequenced treatment alternatives to relieve depression that started like this:
Symptom remission is the desired goal of treatment for depression, given its implications for better daily functioning and better longer-term prognosis. Since no treatment is a panacea, several sequential treatment steps are often needed to obtain remission with a tolerated treatment. If a trial does not result in remission, it is an unsuccessful trial, whether due to lack of efficacy or intolerable side effects, as long as the treatment is vigorously dosed to tolerance and provided for a sufficient duration to achieve remission. The number of treatment steps needed to achieve an adequate benefit is typically used to gauge the degree of treatment resistance, usually with a focus on acute outcomes without reference to longer-term outcomes.
I’d get hung up at the first sentence, "Symptom remission is the desired goal of treatment for depression, given its implications for better daily functioning and better longer-term prognosis." That sentence says that the depression is interfering with their lives, and I would want to say, "What about the people whose lives are the reason they’re depressed?" I’d have trouble with all the other sentences too, but I don’t want to go on and on. If you then told me that the conclusion of the study said:
After two treatment steps, it appears that over 50% of patients will achieve remission if they stay in treatment (i.e., 36.8% step 1 plus 30.6% of the remaining 63.2% of patients). Thereafter, the chances of subsequent remission are much lower. The theoretical cumulative remission rate after four acute treatment steps was 67%.
I would’ve said, "I don’t believe that’s right." I wouldn’t have known what else to say.
Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. by Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M.
Am J Psychiatry. 2006 Nov;163(11):1905-17.
Abstract
OBJECTIVE: This report describes the participants and compares the acute and longer-term treatment outcomes associated with each of four successive steps in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.
METHOD: A broadly representative adult outpatient sample with nonpsychotic major depressive disorder received one (N=3,671) to four (N=123) successive acute treatment steps. Those not achieving remission with or unable to tolerate a treatment step were encouraged to move to the next step. Those with an acceptable benefit, preferably symptom remission, from any particular step could enter a 12-month naturalistic follow-up phase. A score of <or=5 on the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR(16)) (equivalent to <or=7 on the 17-item Hamilton Rating Scale for Depression [HRSD(17)]) defined remission; a QIDS-SR(16) total score of >or=11 (HRSD(17)>or=14) defined relapse.
RESULTS: The QIDS-SR(16) remission rates were 36.8%, 30.6%, 13.7%, and 13.0% for the first, second, third, and fourth acute treatment steps, respectively. The overall cumulative remission rate was 67%. Overall, those who required more treatment steps had higher relapse rates during the naturalistic follow-up phase. In addition, lower relapse rates were found among participants who were in remission at follow-up entry than for those who were not after the first three treatment steps.
CONCLUSIONS: When more treatment steps are required, lower acute remission rates (especially in the third and fourth treatment steps) and higher relapse rates during the follow-up phase are to be expected. Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed.
Here’s the flow chart from that study:
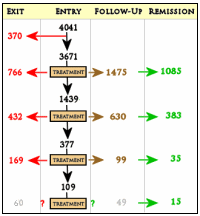
If this is your first time with the STAR*D trial, it’s unlikely that this flow-chart is very clarifying, so I’ve simplified the chart on the right for our perusal, removing the details. In this trial "Exit" means drop-out. After each level of treatment, some number of subjects elect to stop. That group is designated as "Follow-Up" but only a portion of those are in remission [the numbers in gray and green come from Table 3 in the article]. So the 67% is a hypothetical wish in virtual reality, not what the study actually demonstrated. And what of the drop-outs [38% of the subjects who entered some form of treatment dropped out]? In the paper, the authors say:
The cumulative remission rate can be estimated by assuming that 100 patients begin citalopram treatment. Overall, 36.8 will achieve remission in step 1, leaving 63 to proceed to the next step. In step 2, 30.6% (N=19) will remit (.306×63 = 19). In the third step, 13.7% or N=6 will remit (.137 x [100–37-19]). In the fourth step, 13.0% or N=5 will remit. The theoretical cumulative remission rate is 67% (37+19+6+5). Note that this estimate assumes no dropouts, and it assumes that those who exited the study would have had the same remission rates as those who stayed in the protocol.
First off, the numbers at the various levels vary depending on where you look in the paper. The ones I’ve displayed are close, but not guaranteed. Second, I can’t come up with those remission rates [36.8%, 30.6%, 13.7%, and 13.0%]. I’m sure they came from some computation, but I can’t figure out what it was. Here’s how the numbers work out as best I can gather them:
If I use the number that didn’t drop out [3671 – 1427 = 2244], I can get close to what they hypothesize [1516/2244 = 67%]. Since their assumption is absurd ["… assumes that those who exited the study would have had the same remission rates as those who stayed in the protocol"], I calculated 41% [1516/3671 = 41%] considering them as treatment failures. I don’t believe that number either [too high], but for the moment I’d ask why didn’t they report, "the cumulative remission rate was between 41% and 67%" to reflect the best and worst case scenarios from their study?
If you think I’m being too harsh to consider that the people who dropped out of the STAR*D trial were treatment failures, I’m just taking them at their word, "
If a trial does not result in remission, it is an unsuccessful trial, whether due to lack of efficacy or intolerable side effects, as long as the treatment is vigorously dosed to tolerance and provided for a sufficient duration to achieve remission" [ignoring the moralizing at the end]. But you ain’t heard nothing yet. Others have been over this study with a fine tooth comb and found more than enough to write about.
Robert Whitaker, author of
Anatomy of an Epidemic: Magic Bullets, Psychiatric Drugs, and the Astonishing Rise of Mental Illness in America, summarizes their findings in
The STAR*D Scandal: A New Paper Sums It All Up [the new paper being
Efficacy and Effectiveness of Antidepressants: Current Status of Research]. Whitaker’s book, article, and the "new paper" all deserve to be read in the original, but for the moment take a look at what Whitaker summarizes in his article:
1. The STAR*D investigators reported a "cumulative" remission rate of 67% in the abstract of an article, when in fact this was simply a "theoretical" rate.
2. They reported remission rates based on the QIDS-SR scale, even though the pre-specified primary outcome scale was the HRSD, and this switch inflated the remission numbers.
3. They included remission numbers for patients who weren’t depressed enough at baseline to meet study criteria, and thus weren’t eligible for analysis.
4. They reported that 33.3% to 50% of remitted patients relapsed during the 12-year followup, which suggested – when combined with the inflated 67% remitted rate – that perhaps 40% of all patients who entered the trial had recovered and stayed well, when in fact only 3% of the entering patients had a "sustained remission" [and stayed in the trial].
To be honest, these assessments seemed too harsh to me too – but they aren’t. For example, number 2. [changing rating scales in mid-stream] is easy to check out. The
STAR*D Clinical Trial is posted online and a perusal of the
history of changes confirms this conclusion just as it is reported. Chasing down numbers 3. and 4. are equally possible, and I couldn’t find anything to question in their conclusions [In fact, I thought the article,
Efficacy and Effectiveness of Antidepressants: Current Status of Research by Pigott, Allan, Leventhal, Alter and Boren from the Department of Psychology at American University, was exemplary, and, if anything, too soft on the STAR*D, authors considering all the things they found]. There’s more from Dr. Pigott on STAR*D
here.
|
Before responding with my own thoughts about this study, I have to mention this part:
The interactive voice response system collected measures of functioning and quality of life at baseline, 6 weeks, and exit from each acute treatment trial and at monthly intervals during the 12- month naturalistic follow-up phase. Interactive voice response ratings included physical and mental health functioning assessed with the 12-item Short Form Health Survey (SF-12), the 16-item Quality of Life Enjoyment and Satisfaction Questionnaire, and the 5-item Work and Social Adjustment Scale. During the 12-month naturalistic follow-up phase, the interactive voice response also collected monthly QIDS-SR16 scores. The QIDS-SR16 total scores obtained through the interactive voice response system correspond very closely to both the paper-and-pencil QIDS-SR16 and the QIDS-C16.
I find using an interactive voice response system to collect data and rating scales [in fact anything] from patients insulting. They spent $35M of the government’s money and they did their follow-up with one of those annoying phone systems? I’m trying to make nice, but I can’t bring it off with this part. "Press three if you are feeling…?" Give us a break!
|
What I think about STAR*D
I’m not an anti-psychiatrist. I’m a psychiatrist and have prescribed many [but not all] of the drugs mentioned in this study. But I didn’t review the study for this last week or so because I believed what it said or to learn anything about treating depressed people. Nobody who uses these medications and actually sees patients could possibly believe those conclusions. I read it to collect my thoughts about the psychiatrists who wrote it and the government agency that funded it. One of my conclusions was easy – these authors are anti-psychiatrists. Who needs a psychiatrist to collect a list of symptoms, conclude that the person is depressed from that list, then plug the depressed person into an algorithmic protocol like the one they describe? I’m not even sure you would need a doctor or trained mental health professional. An overqualified Walmart greeter I met recently could do that quite well.
As an Internist, I saw lots of depressed people. I didn’t follow the path suggested here even back then. I learned to ask those patients about their lives, their relationships, their experiences. But I didn’t have to ask very much. Just asking anything at all got them started talking and I learned a lot about why they were depressed, but I didn’t know what to do with what I learned – and I wanted to. So I came back and did a Psychiatry Residency to find out what to do, fortunately in the pre-STAR*D days. Had the notions in this study been the curriculum, I would have happily returned to Internal Medicine. Instead, I learned that depression was a human emotion we can all experience. I learned that sometimes, it can be a disease, like in Manic Depressive Illness, or Post-Partum Depression, or when you take certain medicines, etc. But most of the time, it’s a starting place to signal that it’s time to start untying some of those knots that experience has a way of tying in our lives and minds. And that’s what I did for thirty years, learn to help people with the untying. It’s what I wanted to learn back in the Internist days and it was worth the trip.
I gave [and give] the medications. For a few, they’re a godsend. For others, they help to a varying degree. But I never conceived this – "Symptom remission is the desired goal of treatment for depression, given its implications for better daily functioning and better longer-term prognosis." That would mean that feeling depressed was outside the spectrum of human experience, that it said nothing about the life of the person – an alien emotion to be excised. I didn’t believe that as an Internist, and I don’t believe it now. I personally find the whole thrust of this way of looking at people strange. It’s like treating someone with a severe headache with analgesics before finding out if they have Migraine, or a brain tumor, or are having a stroke, or a million other things. I agree that there are people with depression that do have a pathological mood state independent of life/mind things. I used to treat them too when I was up on things. Now, when I see them, I send them to friends who are in the business of treating such cases, who see them all the time. But there aren’t so many in that category.
In other posts, I’ve talked about the sorry state of the Clinical Research Industry and the heavy bias in the Pharmaceutical Industry’s Clinical Trials [the ones Dr. Bernard Carroll calls "experimercials"]. But this study is different with its generous NIMH funding. I’ll even forgo my usual suspicions that the subjects really didn’t really fit the bill diagnostically. But even at that, this study was "fudged" all over the place. The question is why? None of the authors was in the pocket of some drug company whose favorite drug was featured, and yet the whole study reeks of bias. How come? What is the bias of these authors [whom I presume designed this study themselves]?
They seem to have a belief system:
-
Depression, whether it’s a disease or an emotion, is a bad thing and the point is to make it go away with some form of treatment, usually drugs ["Symptom remission is the desired goal of treatment for depression, given its implications for better daily functioning and better longer-term prognosis"].
-
If the treatment doesn’t make it go away, the subject has something called treatment resistance, and should try another treatment ["The number of treatment steps needed to achieve an adequate benefit is typically used to gauge the degree of treatment resistance…"].
-
The goal of Psychiatry is to improve on this treatment scheme ["Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed"].
So one bias in these authors that might explain why they were invested in making this study look better than it was would be to confirm and affirm this idiosyncratic belief system. There’s another related possibility:
Dr. Rush has served as an advisor, consultant, or speaker for or received research support from Advanced Neuromodulation Systems, Inc.; Best Practice Project Management, Inc.; Bristol-Myers Squibb Company; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals, Inc.; Gerson Lehman Group; GlaxoSmithKline; Healthcare Technology Systems, Inc.; Jazz Pharmaceuticals; Merck & Co., Inc.; the National Institute of Mental Health; Neuronetics; Ono Pharmaceutical; Organon USA Inc.; Personality Disorder Research Corp.; Pfizer Inc.; the Robert Wood Johnson Foundation; the Stanley Medical Research Institute; the Urban Institute; and Wyeth-Ayerst Laboratories Inc. He has equity holdings in Pfizer Inc and receives royalty/patent income from Guilford Publications and Healthcare Technology Systems, Inc.
Dr. Trivedi has served as an advisor, consultant, or speaker for or received research support from Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); Bayer; Bristol-Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Johnson & Johnson PRD; Meade Johnson; the National Institute of Mental Health; the National Alliance for Research in Schizophrenia and Depression; Novartis; Parke-Davis Pharmaceuticals, Inc.; Pfizer Inc; Pharmacia & Upjohn; Predix Pharmaceuticals; Sepracor; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories.
Dr. Wisniewski has received research support from the National Institute of Mental Health and served as an advisor/consultant for Cyberonics, Inc.
Dr. Nierenberg has served as an advisor, consultant, or speaker for or received research support from Bristol-Myers Squibb Company; Cederroth; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals Inc.; Genaissance; GlaxoSmithKline; Innapharma; Janssen Pharmaceutica; Lichtwer Pharma; the National Institute of Mental Health; the National Alliance for Research in Schizophrenia and Depression; Neuronetics; Organon, Inc.; Pfizer Inc; Sepracor; Shire; Stanley Foundation; and Wyeth-Ayerst Laboratories.
Dr. Stewart has served as an advisor, consultant, or speaker for or received research support from Eli Lilly & Company; GlaxoSmithKline; Organon USA Inc.; Shire; and Somerset.
Dr. Warden has received research support from the National Institute of Mental Health and has equity holdings in Bristol-Myers Squibb Company and Pfizer, Inc.
Dr. Thase has served as an advisor, consultant, or speaker for AstraZeneca; Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Laboratories, Inc.; GlaxoSmithKline; Janssen Pharmaceutica; Eli Lilly & Company; Novartis; Organon, Inc.; Pfizer Pharmaceutical; Sanofi Aventis; Sepracor, Inc.; Shire US Inc.; and Wyeth Pharmaceuticals.
Dr. Lavori has served as an advisor, consultant, or speaker for or received research support from Bristol-Myers Squibb Company; Celera Diagnostics Inc; Cyberonics, Inc.; the Department of Veterans Affairs; Forest Pharmaceuticals, Inc.; Glaxo-SmithKline; Leaf Cabrezer Hyman and Bernstein; the National Institutes of Health; and Neuronetics, Inc.
Dr. McGrath has served as an advisor, consultant, or speaker for or received research support from Eli Lilly & Company; GlaxoSmithKline; Lipha Pharmaceuticals; the National Institute of Mental Health; the National Institute on Alcohol Abuse and Alcoholism; New York State Department of Mental Hygiene; Organon, Inc.; Research Foundation for Mental Hygiene (New York State); and Somerset Pharmaceuticals.
Dr. Rosenbaum has served as an advisor, consultant, or speaker for or received research support from Astra-Zeneca; Boehringer-Ingelheim; Bristol-Myers Squibb Company; Cephalon; Compellis; Cyberonics; EPIX; Forest; GlaxoSmithKline; Janssen; Lilly; MedAvante; Neuronetics; Novartis; Orexigen; Organon; Pfizer, Inc; Roche Diagnostics; Sanofi; Schwartz; Somaxon; Somerset; Sepracor; Shire; Supernus; and Wyeth. He has equity holdings in Compellis, Medavante, and Somaxon.
Dr. Sackeim has served as an advisor, consultant, or speaker for or received research support from Cyberonics, Inc.; Eli Lilly & Company; Magstim Ltd.; MECTA Corporation; Neurocrine Biosciences Inc.; Neuronetics Inc.; NeuroPace Inc.; and Pfizer Inc.
Dr. Kupfer has served as an advisor, consultant, or speaker for or received research support from Amersham; the Commonwealth of Pennsylvania; Corcept Corporated; Eli Lilly & Company; F. Hoffmann-La Roche Ltd.; Forest Pharmaceuticals; Lundbeck; the National Institute of Mental Health; Novartis; Pfizer, Inc; Servier Amerique; and Solvay/Wyeth. He has equity holdings in B

ody Media and Med Avante and receives royalty income from Oxford University Press.
Dr. Fava has served as an advisor, consultant, or speaker for or received research support from Abbott Laboratories; Alkermes; Aspect Medical Systems; Astra-Zeneca; Bayer AG; Biovail Pharmaceuticals, Inc.; BrainCells, Inc.; Bristol-Myers Squibb Company; Cephalon; Compellis; Cypress Pharmaceuticals; Dov Pharmaceuticals; Eli Lilly & Company; EPIX Pharmaceuticals; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals Inc.; GlaxoSmithKline; Grunenthal GmBH; J & J Pharmaceuticals; Janssen Pharmaceutica; Jazz Pharmaceuticals; Knoll Pharmaceutical Company; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck; MedAvante, Inc.; Novartis; Nutrition 21; Organon Inc.; PamLab, LLC; Pfizer, Inc; PharmaStar; Pharmavite; Roche; Sanofi/Synthelabo; Sepracor; Solvay Pharmaceuticals, Inc.; Somerset Pharmaceuticals; and Wyeth-Ayerst Laboratories. He has equity holdings in Compellis and MedAvante.
Dr. Niederehe, Dr. Lebowitz, and Mr. Luther report no competing interests.
Pitiful!…
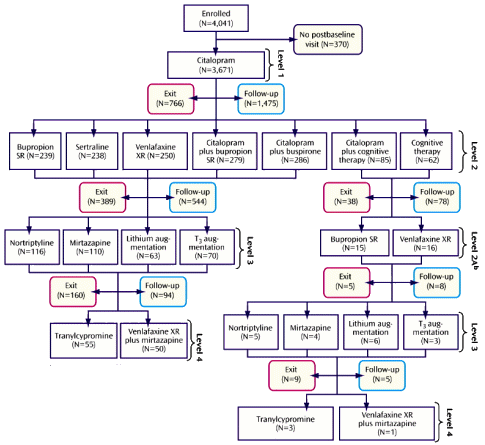
 If this is your first time with the STAR*D trial, it’s unlikely that this flow-chart is very clarifying, so I’ve simplified the chart on the right for our perusal, removing the details. In this trial "Exit" means drop-out. After each level of treatment, some number of subjects elect to stop. That group is designated as "Follow-Up" but only a portion of those are in remission [the numbers in gray and green come from Table 3 in the article]. So the 67% is a hypothetical wish in virtual reality, not what the study actually demonstrated. And what of the drop-outs [38% of the subjects who entered some form of treatment dropped out]? In the paper, the authors say:
If this is your first time with the STAR*D trial, it’s unlikely that this flow-chart is very clarifying, so I’ve simplified the chart on the right for our perusal, removing the details. In this trial "Exit" means drop-out. After each level of treatment, some number of subjects elect to stop. That group is designated as "Follow-Up" but only a portion of those are in remission [the numbers in gray and green come from Table 3 in the article]. So the 67% is a hypothetical wish in virtual reality, not what the study actually demonstrated. And what of the drop-outs [38% of the subjects who entered some form of treatment dropped out]? In the paper, the authors say:
 ody Media and Med Avante and receives royalty income from Oxford University Press.
ody Media and Med Avante and receives royalty income from Oxford University Press.
You might like this poster from the people at the Icarus project. It has nothing to do with pharma but everything to do with people and depression.
http://raisingbipolar.com/2011/03/11/suicide-ideation-a-chance-to-make-a-life-change/
The graphic you found to insert at the end of this post says a lot about the issues don’t it? I can almost smell the rank odor of stale urine in one of those country road gas stations where they long ago gave up keeping the men’s room locked because of the implied expectations – that if they bothered to lock it, well someone might expect it to be clean.
You might call STAR*D a $35 million folly. For a while, STAR*D was the darling of NIMH, but nowadays it’s a sad old thing. It’s as though nobody is minding the STAR*D store any more. The STAR*D page on the NIMH website hasn’t been updated in over 3 years – since 25 February 2008. The STAR*D page on ClinicalTrials.gov hasn’t been updated since 24 September 2009.
Worse, no results have been posted on the ClinicalTrials.gov site – none! There is, to be sure, a listing of at least 64 publications in scientific journals, which is testimony to the self interested diligence of those in academia who rode the wave of STAR*D in the cause of their professional advancement. But these 64 publications are all over the map, and nobody within NIMH has had the wit and gumption to pull together a coherent synthesis of the findings of this expansive enterprise. The scientific bureaucrats who were swanning about within NIMH, basking in the reflected glory of STAR*D a few years ago, owe at least this much to John Q. Public, who footed the bill.
It gets worse. The STAR*D data bases are now being used for very complicated, very technical, very expensive genome wide association studies of the genetics of depression. Don’t hold your breath, anybody. As the iconic academic psychologist Joseph Wolpe said many years ago, outcome studies on mixed pathologies are of very little value. And drilling down into the genetics of highly heterogeneous clinical data sets like STAR*D is a recipe for futility. So why do they keep on perseverating? Because the name of the game is to keep the game going, that’s why. And NIMH is complicit to a fare-thee-well.
The entire STAR*D effort resembles what Richard Feynman called Cargo Cult science. They went through all the right moves but no planes landed.
This is pretty close to the treatment guideline stuff for cardiologists. It was multipronged:
1. Establish that the goal of all treatment for depression is to have complete alleviation of symptoms (there are a lot of papers and guidelines out there that said that). Reasons?
1. People stay on antidepressants longer
2 It lowers the bar for what is treatable. ie, if complete resolution of symptoms means not depressed, then any symptoms means depressed, ie to be treated.
This has the added advantage that need for medication is not an independent clinical judgment. It becomes an algorithm (any symptom = need to treat)
The endless algorithm also took advantage of what was widely believed to be true, whether or not it was: a third of depressed patients get better on any treatment, a third get worse, and a third stay the same.
Four levels of treatments was designed to not just “catch” everybody in the hope of finding something effective. It also took advantage of the assumption that some people would just naturally get better. If they were still taking a drug, it would look like a drug benefit..
This same kind of thinking was what drove the development of guidelines for chronic, long-term use of antidepressants. If you tell people “it” is chronic, and that any symptom is a sign of “it”–then the appearance of any symptom at any time proves them right, which justifies long-term treatment.
This is not an exaggeration of the thinking.
So it was a two-fer.
Micky: I’m thrilled that you are now researching, reflecting, and posting on STAR*D.
Checking for new posts from you, and reading your blog, has become part of my morning ritual ever since I stumbled on it several months ago. I deeply appreciate both the quality of your research and your cut-to-the chase perspective on it.
I particularly liked your Seroquel, Clinical Trials, “Evidence-Based†Medicine exposes and it was through your blog on the latter that I learned about Trivedi’s first attempt at computerized care.
For 5+ years now, I’ve been obsessed with deconstructing STAR*D by comparing its published methods and findings with STAR*D’s pre-specified research measures and analytic plan as described in primary source documents (e.g., the NIMH-approved STAR*D Research Protocol, STAR*D Clinical Procedures Manual, STAR*D Patient Education Manual, and their 2004 Controlled Clinical Trials article).
If interested, please send me an email at: pathware@erols.com and I will send you my recently published article “STAR*D: A Tale and Trail of Bias†as well as some other information that you might want to incorporate into your analysis.
Many, many thanks for your blogging efforts,
Ed
Ps: You are 100% correct. STAR*D’s authors are the true “anti-psychiatrists.â€
Not to be a bore but this whole “goal of treatment” stuff is pretty important. Here’s your old buddy Nemeroff from 2001:
Remission of symptoms is the treatment goal increasingly accepted by the medical community, explained one of three discussion leaders at the roundtable, Charles B. Nemeroff, M.D., Ph.D., Reunette W. Harris professor and chairman of the Department of Psychiatry and Behavioral Sciences at Emory University School of Medicine in Atlanta.
Remission aims for the virtual elimination of a person’s symptoms of depression or anxiety and restoration of his or her psychosocial and occupational functioning – essentially allowing a return to enjoying life and making strides toward recovery, he added. A person achieves remission when symptoms are absent for at least six months. In contrast, response, or only partial improvement of symptoms, is a common standard of care.
This was part of a “new paradigm of care” discussed:
New depression and anxiety treatment goals defined
Mental health leaders reconcile provider and consumer expectations
http://www.eurekalert.org/pub_releases/2001-12/pn-nda120301.php
The goal was the stepped treatment you described, which had to be justified and was in the article cited above:
In addition to the need for mental health professionals to communicate that recovery is possible, stated the participants, consumers must take ownership of their illness and treatment, challenging their providers to explore different options if recovery is not being achieved.
In other words, “add on.”
Here’s the goal described in a patient piece from Wyeth (long term goal “to prevent it from returning,” ie, chronic antidepressants):
http://www.kemperdrug.com/downloads/depression%20remission.pdf
Thanks to all for the comments. STAR*D encapsulates all the contoversy about the phamacotherapy of depression. They designed a study to clear things up, and 70+ papers later, there sure is no clarity coming out of their results. Were they just sloppy, or were they being slippery because things were not going as planned? I’m in the middle of Ed Piggot’s STAR*D: A Tale and Trail of Bias [above]. I’d suggest anyone interested write him for a copy and check out the primary documents he’s posted here. He says he’s “obsessed with deconstructing STAR*D.” From what I’ve read so far, somebody needs to be obsessed about it. He’s onto some pretty damning evidence that suggests a level of data manipulation that’s worthy of a full investigation by the NIMH, maybe even an investigation of the NIMH.
Something’s rotten in the State of Denmark…
In 2007 I wrote this article on StarD that agrees with much of what you say: http://www.scribd.com/doc/52371531.
When I read APA’s 2007 press release about the STAR*D study results, I thought the 67% remission rate was too high. Carlat’s article confirmed my suspicions. I naively contacted the APA, and they brushed me off.
Nice column. there are very few colleagues as of 2011 who believe in the biopsychosocial model anymore, not that this is the only paradigm to treat people by, but, it works for me, moreso for my patients who look at the multifactorial causes and impacts on psychiatric disorders/struggles. As you wrote in a more recent column, I sense there are still enough of us who do preach this mentality that annoys big pharma, who are pushing for non-psychiatrists to take over prescribing habits, so these colleagues above you give their real curricula vitae, who are probably about to retire anyway and don’t care how they impact on our profession, will take the money and literally run.
By the way, any opinion why the APA is having their alleged professional conference in Hawaii next month? To avoid the usual backlash by the antipsychiatry paparazzi?
Or, to just keep those who are not minions and mindless supporters from attending?
Doesn’t affect me, dropped my membership 15 years ago when I saw the real nature of this organization’s agenda front and center in D.C.