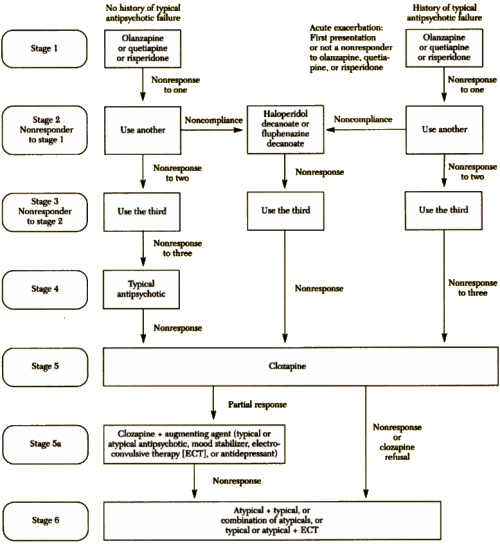
I know that I’m bad to post graphs, charts, table, flow-sheets, etc., but this one is special. It’s the algorithm for treating Schizophrenia from the Texas Medication Algorithm Project in 1999, and it was as outrageous as they’ve said it was:
The Texas Medication Algorithm Project: Development and Implementation of the Schizophrenia Algorithm
by John A. Chiles, M.D., Alexander L. Miller, M.D., M. Lynn Crismon, Pharm.D., A. John Rush, M.D., Amy S. Krasnoff, M.A. and Steven S. Shon, M.D.
Psychiatric Services 50:69-74, January 1999
New York Times
By MELODY PETERSEN
February 01, 2004The drug industry has created vast markets for products like Viagra, Celebrex and Vioxx by spending billions of dollars on consumer advertising. But to sell medicines that treat schizophrenia, the companies focus on a much smaller group of customers: state officials who oversee treatment for many people with serious mental illness. Those patients – in mental hospitals, at mental health clinics and on Medicaid – make states among the largest buyers of antipsychotic drugs. For Big Pharma, success in the halls of government has required a different set of marketing tactics. Since the mid-1990’s, a group of drug companies, led by Johnson & Johnson, has campaigned to convince state officials that a new generation of drugs – with names like Risperdal, Zyprexa and Seroquel – is superior to older and much cheaper antipsychotics like Haldol. The campaign has led a dozen states to adopt guidelines for treating schizophrenia that make it hard for doctors to prescribe anything but the new drugs. That, in turn, has helped transform the new medicines into blockbusters.
Ten drug companies chipped in to help underwrite the initial effort by Texas state officials to develop the guidelines. Then, to spread the word, Johnson & Johnson, Pfizer and possibly other companies paid for meetings around the country at which officials from various states were urged to follow the lead of Texas, according to documents and interviews that are part of a lawsuit and an investigation in Pennsylvania.
How did this play out? In May 2001, as Pennsylvania was weighing whether to adopt the Texas guidelines, Janssen Pharmaceutica, a Johnson & Johnson subsidiary that sells Risperdal, paid $4,000 to fly two state mental health officials to New Orleans, where they dined at an elegant Creole restaurant in the French Quarter, visited the aquarium and met with company executives and Texas officials, according to documents. Janssen also paid two Pennsylvania officials $2,000 each for giving speeches at company-sponsored educational seminars for doctors and nurses working in the state’s prisons. The payments were discovered a little more than a year ago by Allen L. Jones, an investigator in the inspector general’s office in Pennsylvania, who stumbled upon them when he was looking into why state officials had set up a bank account to collect grants from pharmaceutical companies.
A Lone Wolf Takes on the Drug Leviathan
An interview with Allen Jones
The Rutherford Institute: Oldspeak
By John W. Whitehead
10/13/2005When Allen Jones was appointed lead investigator in July 2002 in a case concerning off-the-books payments from pharmaceutical companies, he had no idea that his discoveries would cost him his career and propel him to the core of President George W. Bush’s national drug policies. An investigator for the Pennsylvania Office of the Inspector General (OIG), Jones’ findings in the case showed that the drug company Janssen had paid honorariums to key state officials who held significant influence over the prescriptions issued for state institutions such as prisons and mental health hospitals. Although the accounts receiving these payments were marked for “educational grants,” funds were being channeled to state employees who developed guidelines recommending new, more expensive drugs rather than older, cheaper drugs with safe, proven effects. These companies were influencing officials with trips, perks and lavish travel accommodations as a means of inducing the officials to endorse their products. Jones discovered that one of the new drugs being recommended, Risperdal, has been shown to have potentially lethal side effects such as ketoacidosis, coma and possibly death.
After initially revealing his discoveries to OIG managers, Jones was taken off the case but told that he could pursue it on his own. In the words of the OIG supervisor who took Jones off the case and participated in threatening him, “Drug companies write checks to politicians… on both sides of the aisle.” When Jones went public with his findings, he was escorted out of his workplace and told not to reappear on OIG property…
Company Accused of Improprieties in Marketing Risperdal
by Jim Rosack
Psychiatric News [2007] 42(3):30The Texas attorney general says TMAP was just one part of an elaborate marketing scheme to increase psychotropic drug sales. The Texas state attorney general joined a whistleblower lawsuit this past December accusing the pharmaceutical and consumer goods giant Johnson and Johnson inc. of exaggerating the benefits and minimizing the known adverse effects associated with its second-generation antipsychotic Risperdal [risperidone], marketed by subsidiary Janssen L.P. The suit further alleges the company and its subsidiaries “improperly influenced” at least one Texas state mental health program official through the payment of “substantial financial contributions” aimed at ensuring a preferred position for Risperdal during the development and implementation of the Texas Medication Algorithm Project (TMAP).
The lawsuit was originally filed in 2004 by Allen Jones, a former employee in Pennsylvania’s Office of the Inspector General (OIG). as an OIG investigator, Jones had investigated allegations of impropriety during Pennsylvania’s efforts to implement PENNMAP, a slightly modified version of TMAP. As a result of Johnson and Johnson’s alleged improper influence of state officials through illegal payments of significant sums of money, the lawsuit claims that Risperdal became a widely prescribed “preferred” first-line medication in the TMAP and PENNMAP algorithms for the treatment of schizophrenia.
To assure Risperdal a first-line spot in the algorithms, the suit alleges that Johnson and Johnson overstated Risperdal’s effectiveness in treating patients with schizophrenia and downplayed the drug’s side effects. The suit states that the company also manipulated data collected during development of TMAP, so that Risperdal would appear to be more effective and safer than it actually was. As a result of Risperdal’s preferred position in TMAP, the state mental health and Medicaid programs were said to have paid “dollars per pill” for Risperdal when it could have paid “pennies per pill” for generic first-generation antipsychotics that were equally effective. Neither Johnson and Johnson nor Janssen responded to inquiries by Psychiatric News for this article.Texas Attorney General Greg Abbott was quoted by the Austin American-Statesman newspaper as saying, “We believe Texas has been defrauded of some money, and we’re going to be looking to get our money back”…
Court Ruling Clears Way for Jury Trial in $1 Billion Texas Medicaid Whistleblower Lawsuit
Johnson and Johnson
Stockhouse: AUSTIN, TX
March 04, 2011A recent state district court ruling has cleared the way for a jury to hear claims filed by the State of Texas and plaintiff Allen Jones based on allegations that pharmaceutical manufacturer Janssen L.P. used false marketing tactics to convince state officials to spend millions on a schizophrenia drug. The ruling was issued late Thursday, March 3, 2011, in Judge John Dietz’ 250th District Court in Travis County following summary judgment motions filed by both the State of Texas and Janssen, a division of New Brunswick, N.J.-based Johnson & Johnson.
The original complaint was filed in 2004 based on evidence uncovered by Mr. Jones during his work as an investigator with the Pennsylvania Inspector General’s Office. The lawsuit says Janssen engaged in a systematic and wide-ranging scheme to convince state Medicaid officials to give preferential treatment to the company’s Risperdal schizophrenia medication. The drug was no better and no safer despite being substantially more expensive than older medications that treat the same illness, the lawsuit alleges. Janssen worked to build revenue by actively and purposefully marketing the powerful antipsychotic drug for use in children, the lawsuit says, even though the medication was approved only for the very narrow purpose of treating adult schizophrenia. In the years since Risperdal was first introduced, Texas has paid more than $500 million for the drug. In the order issued in The State of Texas, ex rel Allen Jones v. Janssen, L.P., et al., No. D-1-GV-04-001288, the Court ruled against Janssen on two motions that would have ended the case, and ruled in favor of the state on three summary judgment motions.
"We are very pleased that a Texas jury finally will be able to scrutinize Janssen’s actions, which we allege have unfairly cost the state’s taxpayers hundreds of millions of dollars for a drug that was no better than older, cheaper medicines," says Dallas attorney Tom Melsheimer, who represents Mr. Jones with Austin attorney Tommy Jacks. "The defendants fought tooth-and-nail to keep this case from a jury, and that effort has failed." The defendants’ total exposure in the anticipated jury trial, currently set for June 21 in Austin, exceeds $1 billion, including damages, penalties, and other potential liabilities, Mr. Melsheimer says.
-
Allen Jones Statement [2004]
-
The Lawsuit [as of 2011]

Sorry, the comment form is closed at this time.