Janssen® Connect™ Launches In California
New Service Helps Practitioners Coordinate Care for Patients Receiving Company’s Long-Acting Injectable Therapies
Titusville, NJ (January 31, 2011) – Janssen®, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc., announced today the launch in California of Janssen® Connect™, a new service for psychiatrists and other healthcare professionals and their patients who have been prescribed a Janssen® long-acting injectable atypical antipsychotic medicine. These medicines are INVEGA® SUSTENNA® (paliperidone palmitate), for the treatment of schizophrenia, and RISPERDAL® CONSTA® (risperidone), for the treatment of schizophrenia and longer-term treatment of Bipolar I Disorder.
Janssen® Connect™ is a service designed to help make it easier for patients to access Janssen® long-acting injectable therapies. Janssen® partners with treatment teams that request to be a part of the program and works directly with them to provide their patients with support and resources, including: alternate injection sites of care, such as pharmacies; trained healthcare professionals to provide injections; facilities that may be more conveniently located for patients; access and reimbursement services; appointment reminders; and scheduling of future injection appointments.
“We are pleased to offer this program to prescribers and patients, as part of our long-standing commitment to people with mental illness,” said Denice Torres, president of Janssen®.
The move from an inpatient to an outpatient setting may be complicated for some people with mental illness, and this transition is critical to the process of recovery. Janssen® Connect™ assists with this transition by keeping inpatient and outpatient healthcare professionals and planners informed about timing of injections, doses of medication, adverse events or patient concerns. Healthcare professionals also will be notified if a patient has missed an appointment or was not given medication for any reason, which can help identify situations in which a physician may need to intervene.
Janssen® Connect™ reimbursement-support services and care-coordination services are provided as a service by Proherant Health Inc., under contract for Janssen®, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc. These support services are made available as a convenience to patients, and no additional compensation is required from or paid to patients or prescribers. Proherant Health, Inc. and other providers are responsible for the services they provide.
Incidence of extrapyramidal symptoms and tardive dyskinesia in schizophrenia: thirty-six-month results from the European schizophrenia outpatient health outcomes study.
Lilly Research Centre, Eli Lilly and Company, Windlesham, Surrey, United Kingdom.by Novick D, Haro JM, Bertsch J, and Haddad PM.Journal of Clinical Psychopharmacology. 2010 30(5):531-40.
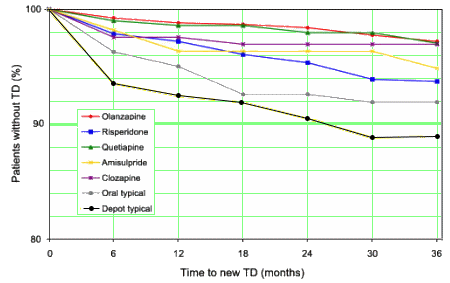
The incidence of treatment-emergent extrapyramidal symptoms (EPSs) and tardive dyskinesia (TD) in schizophrenic patients, and the clinical characteristics associated with an increased risk of developing EPSs and TD were examined. Patients (N = 7728) in the 3-year, prospective, observational Schizophrenia Outpatient Health Outcomes study were examined according to baseline antipsychotic drug exposure. At baseline, 4893 patients (63.3%) had no EPS, and 6921 (89.6%) had no TD. Extrapyramidal symptoms and TD were assessed separately during follow-up: frequency and time to appearance from Kaplan-Meier survival curves and factors associated with time to appearance using Cox proportional hazard regression models. The cumulative incidence of EPS ranged from 7.7% (olanzapine) to 32.8% (depot typical drugs). Compared with olanzapine, patients taking depot typical drugs, oral typical drugs, risperidone, and amisulpride had a significantly higher risk of developing EPS. Differences from clozapine were marginally significant. High baseline clinical severity was associated with a significantly higher risk of developing EPS. The incidence of TD ranged from 2.8% (olanzapine) to 11.1% (depot typical agent). Compared with olanzapine, patients taking depot typical agents, oral typical agents, and risperidone had a significantly higher risk of developing TD. Baseline factors associated with a significantly higher risk of developing TD were age, EPS, a higher negative Clinical Global Impression score, and presence of gynecomastia. In summary, patients treated with typical antipsychotic agents (oral and depot) and risperidone had a higher risk of developing EPS and TD than patients treated with olanzapine. Higher baseline clinical severity was associated with EPS development, whereas age, presence of EPS, a higher negative Clinical Global Impression score, and presence of gynecomastia were associated with TD development.
From Eli Lilly and Company, Windlesham, Surrey, United Kingdom; †Departament de Psiquiatrı´a, Universitat Auto`noma de Barcelona, Spain; ‡Sant Joan de De´u-SSM, CIBERSAM; §Sant Joan de De´u-SSM, Fundacio Sant Joan de De´u, Sant Boi, Barcelona, Spain; and ||Greater Manchester West Mental Health NHS Foundation Trust and University of Manchester, Manchester, United Kingdom.
Received September 14, 2009; accepted after revision July 9, 2010.
Reprints: Diego Novick, MD, Lilly Research Centre, Eli Lilly and Company,
Erl Wood Manor, Sunninghill Rd, Windlesham, Surrey, GU20 6PH, United Kingdom
The Schizophrenia Outpatient Health Outcomes study was funded by Eli Lilly and Company.
Copyright * 2010 by Lippincott Williams & WilkinsAUTHOR DISCLOSURE INFORMATION
Diego Novick is a Lilly employee. Josep Maria Haro has acted as a consultant, received grants, or acted as a speaker in activities sponsored by the following companies: AstraZeneca, Eli Lilly, GlaxoSmithKline, and Lundbeck. Jordan Bertsch was a statistical consultant for the SOHO study. Peter M. Haddad received honoraria for lecturing and consultancy from the manufacturers of several antipsychotic agents including AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Janssen-Cilag.
I want to linger for a moment on that ad [“no is the beginning of yes”…]. It’s not an ad for their products [INVEGA® SUSTENNA® and RISPERDAL® CONSTA®]. It’s not even an ad for the services in that announcement up there [Janssen® Connect™]. It’s an ad for ways a doctor can get patients to use their products. In the third video [Importance of the Therapeutic Alliance], we’re told how to get to know our patients, gain their trust, then use it to "guide the patient" into using these injectables – "use the therapeutic alliance" they say. They even quote an APA blurb about the Therapeutic Alliance [see top graphic]. If I may allow my inner analyst to speak, that really pisses me off. The concept of the Therapeutic Alliance was Freud’s, though it was named later by Edward Bibbering. It is an alliance between the patient’s healthy ego and the analyst against the destructive forces in the patient’s mind. It is not make friends with the patients so they’ll trust you and do what you want them to do. And speaking of trust, why does the psychiatrist want the patient to use Long Acting Therapy? It’s because the psychiatrist doesn’t trust the patient to take the medication. Freud’s and Bibbering’s Therapeutic Alliance is built on mutual trust, hard earned. Janssen’s Therapeutic Alliance is a con job. So Janssen’s marketing target is psychiatrists, not patients – in fact, it’s doctors who see their task as keeping Schizophrenic patients medicated by making friendly [and apparently need to be taught how to do that].
Answer to your last question: Patent extenders, profit-builders to start
[The therapeutic alliance]”It is not make friends with the patients so they’ll trust you and do what you want them to do. And speaking of trust, why does the psychiatrist want the patient to use Long Acting Therapy? It’s because the psychiatrist doesn’t trust the patient to take the medication.”
That’s exactly what it was in my experience as a victimized & vulnerable patient. As soon as I figured that out, I complied exactly to the extent that I could win my release & effect an escape from that abuse. I even stated to them that we were in an adversarial relationship – I know they heard that because they documented it. What an idiotic fool I was – and that was after admitting to the bogus interrogators who referred to themselves as a treatment team that as a part of the trauma I experienced that I didn’t think I would ever develop the capacity to trust again. They certainly put the final nail in that coffin.
They taught me to be entirely skeptical and distrustful of each and everything they said and did. They undermined their profession, and they destroyed their own treatment aims by doing this. I didn’t realize the extent of the dishonesty, manipulation and deceit until I finally got my medical records. What was in the record was breathtakingly malpractice. It was malicious. It was harmful, and it is wrong.
When I worked with abused animals, a wise animal trainer told me to “never smack the pup on the nose after it finally responds to your command to come after it misbehaves if you ever want it to approach you again.” The way forward was to give the animal praise for approaching, no matter the length of time to affect that. Reward the desired behavior; ignore the undesired. Basic, basic, basic. Find something the animal likes and use that as a beginning reward. Start small & specific and incrementally increase and generalize the rewarded desired behavior. Consistency, gentleness, reliability, mutual understanding and patience underpin building trust.
When, Dr. Nardo, does this finally get called what it is – profession-wide malpractice? When will those licensed as professionals – those who are charged with upholding the social contract and self-regulating their – your – profession – actually going to do so?
You mentioned in your earlier post about serving in the clinic that as part of your routine, you corrected treatment for wrong diagnoses. When will those who misdiagnosed and prescribed harmful or ineffective treatments be held accountable? Where is the peer review and the sanctioning of those who do not provide minimal standards of care and practice? In a prior comment, I suggested that a modern day Flexner Report is needed. Perhaps a new professional organization – let’s call it Psychiatrists for Human Rights – might be established with its mission to examine the mission and social contract of psychiatry, examine medical education – pre and post licensure, and then affirm minimally acceptable standards of psychiatric care and practice. That would be something. Declare independence from payers, hospitals, pharma, device makers – anyone outside the psychiatrist and patient is intentionally excluded from the determination of professional scope of practice.
Real people are being harmed, and real people are suffering and dying needlessly as a result of this.