In medical school, statistics wasn’t the most popular of courses, but as a math major, I thought it was pretty wonderful [having taken the other kind of math]. The professor was really good, but long suffering. Years later, I was in a research fellowship taking a Master’s in statistics, and he was one of the teachers. He remembered me as one of the few who was engaged in medical school. He saw that I could get lost in all the swell tests one could do, particularly with the new-fangled computers [then they filled rooms]. And he countered all the number play fun by making us display the data in the rawest possible form first. He said, if it’s not there in the raw form, watch out! The saying was, "Statistics will tell you anything if you torture them long enough." I’m wary of all the manipulated graphs and tables that populate our literature. Are they hiding something? creating something? putting ‘lipstick on a pig’?, ‘making a silk purse out of a sow’s ear’? So for all the wonderful subtlety that statistics can reveal, I still think looking at the raw data is always the first step…
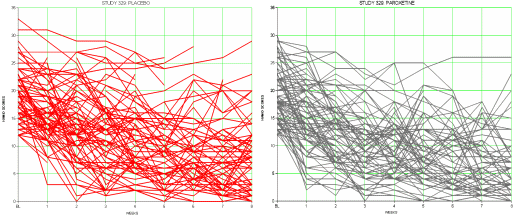
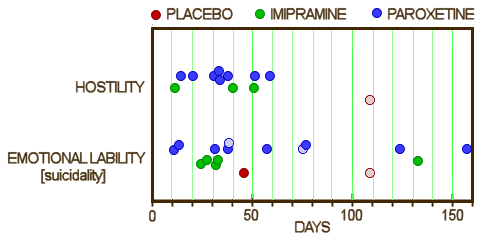
This series of posts [the lesson of Study 329: …] is really only about one thing, the fact that the data for Study 329 wasn’t made available publicly for over a decade, in spite of innumerable articles, posters, meta-analyses, debates, accusations, and million [now billion] dollar lawsuits. The reviewers for JAMA and the JAACAP quickly saw that Study 329 hadn’t met it’s Primary Outcomes, as did Jon Jureidini and Leeman McHenry. They had no way to directly see the Adverse Reaction Data. But they couldn’t overcome the JAACAP editor, Dr. Mina Dulcan, the Psychiatric Establishment, the Manufacturer, or the authors until the FDA, the US Senate, and the DOJ got involved. And the paper still hasn’t been retracted. I don’t know absolutely that having this raw data up front would’ve made things easier, but it’s hard to imagine it wouldn’t.
My apologies for not realizing GSK had posted the adverse reaction appendices on their site. Do you have access to the 2006 GSK authored (along with one Israeli scientist I think) review paper of the safety data (from all 3 Paxil MDD adolescent studies I think). Do you have a sense of what, of anything, was missing from that presentation of the data now that you have fuller access to the data. That is the review Keller appeared so concerned In 2004 would seem to be different from what was presented in the 2001 paper.
Evaluation of Suicidal Thoughts and Behaviors in Children and Adolescents Taking Paroxetine
by Alan Apter, M.D., Alan Lipschitz, M.D., Regan Fong, Ph.D., David J. Carpenter, M.Sc., Pharm.D., Stan Krulewicz, M.A., John T. Davies, M.Sc., Christel Wilkinson, M.Sc., Philip Perera, M.D., and Alan Metz, M.D.
JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY 2006 16[1/2]:77–90.
Do you have a sense of what, of anything, was missing from that presentation of the data now that you have fuller access to the data.
Not yet, but I’m thinking about it. I’m not sure this paper is the same as the one they were emailing about. It might be a version modified by their complaints. It ends with:
“We are grateful to Karen Wagner, M.D., and Neal Ryan, M.D., for their assistance in reviewing and classifying the blinded case narratives…”
Calls for retraction of the 2001 paper continue to be unsuccessful. The message from JAACAP is that Dulcan was acting within the limits of her editorial purview. Also, that no scientific errors were made.
Looking at your letter to Andres Martin your appear, like others, to attempt to emphasize the singular nature of Study 329.
Why not turn this around?
JAACAP/AACAP’s has voiced no criticsm that I am aware of about Study 329. Their approach to Dr. Dulcan was to renew her term in 2002 and call her in 2007 at the end of her 10 years of tenure an editor extraordinaire. Dr. Ryan in 2011/2012 was appointed an “AACAP Distinguished Fellow” reserved for those who perform far beyond adequacy and in 2008 was part of their consensus panel on establishing ethical guidelines regarding conflicts of interest with industry. Dr. Wagner in 2007 was recruited by AACAP to help write their most recent official guidance on the treatment of depression in adolescents.
So why not turn this around?
Why assume that Study 329 is so special? We don’t have access to the raw reviewer’s comments on any of the other papers on which she acted within the standard editorial guidelines of JAACAP of her tenure from 1997-2007. We don’t have access to the correspondence between Wagner, Ryan, Keller and other coauthors, or between them and drug companies, on any of their other papers in JAACAP from that time period. AACAP in 2007/2008 engaged all of them as active participants.
So follow that to its logical conclusion.
Study 329 reflected standard practice in child psychiatry research engaged in by those primary authors and reflected the editorial standards of Dr. Dulcan when she oversaw the journal from 1997-2007. Then continue to use the information we have available on the 2001 JAACAP article to illustrate what that standard practice was. It’s the only one where we have as much data that allow the curtain to be pulled back.
Stop believing it is so singular.
It says more about the integrity of JAACAP/AACAP’s voice if it wasn’t.
So join them in that view and then see how they respond.
AACAP’s 2007 Practice Parameters on Depression in Adolescents (the previous one had been 1998) don’t reference the 2001 paper. A paper Dr Wagner had been giving multiple presentations on through 2002 and one that Dr. Ryan had referred to as a landmark paper in the field.
The 2001 Keller paper reflects the standards of JAACAP for the handling and presentation of data during Dr. Dulcan’s tenure.
Keep voicing that, and keep making clear what those standards were.
Then send an open letter to AACAP and see if they want to present any reason to disagree.
In other words, trying to force the retraction put the onus on you.
Agreeing that the paper is a fair representation of the journal’s level of scholarship is different.
I would hope they would choose to voice disagreement with that.
“19. Study 329 did not show that Paxil was more effective than a placebo on either of it’s primary end points or any of it’s predefined secondary endpoints.
20. The 329 Study investigators later added several additional efficacy measures not specified in the protocol. Paxil separated statistically from placebo on certain of these measures.”
It would be sad if they would not voice disagreement with that being representative of the quality of work presented in the journal. So often, the abstract is all people see of a paper. There is usually the expectation that the senior editor, and by extension the journal, would demand more.
Maybe I’m wrong 1BOM. The adverse reaction data is important, but even those jaded would have a hard time reading that, then reading the abstract, and not be impressed.
That’s not just not good enough for the FDA. That is impressive on its own merits. That is something easily grasped.
It went to JAMA. SKB considered it going to AJP. As the DOJ complaint states, it’s not that they were going to a more specialized journal because of the population they were studying: “Given the comments received, GSK and the lead author decided to revise the article and send it to what they called “a less demanding journal.”
When a physician reads an article’s abstract, perhaps they should think less about the impact factor, and more about whether it is “demanding.”
Forget ghostwriting, does JAACAP have any interest in expressing regret that they published a paper whose abstract purports to show effectiveness when every predefined primary and secondary endpoint did not achieve statistical significance. And which is now the prime exhibit in a 3 billion settlement between GSK an the feds?
Again, forget retraction. If they don’t even consider that unfortunate. If that’s just how science goes and was the right call given the available information of the time.
Forget the retraction.
Make the point that this abstract/paper is fully consistent with JAACAP’s standards. There are a lot of things a journal can do short of retraction.
Then let their silence speak within that context.
“Today brings to resolution difficult, long-standing matters for GSK. Whilst these originate in a different era for the company, they cannot and will not be ignored. On behalf of GSK, I want to express our regret and reiterate that we have learnt from the mistakes that were made.” – GlaxoSmithKline CEO Sir Andrew Witty
“We will work with you to modify the current paper, but reserve the judgment to renew out argument for 3 papers if we are unable to fix this one to our satisfaction. A critical issue will be whether working together we will be able to explain in detail what is different here than in 329 about 329, annd in the presentation about the 2 other studies, so that it is 100% clear in this paper that there is no way to read it and think that 329 is being criticized and that it was not written with complete integrity and accuracy given the data we had and should have had as investigators, on the part of investigators and our collegues from SK who worked on it.” – initial draft of message from Keller to GSK
Q: “Do you have no regrets about publishing the study?”
“I don’t have any regrets about publishing at all. It generated all sorts of useful discussion which is the purpose of a scholarly journal,” Dulcan said.
“Also, she has been the pathfinder, steward, and trail maker for the science of child and adolescent psychiatry during a period of rapid growth and great change. … She was diligent and discerning as well as innovative. Her decisions and leadership were always thoughtful and measured. She chose her battles well, stood her ground, and let the science shine through.” – Virginia Q. Anthony
The universities will likely not listen.
Funding agencies will likely not listen.
That is really all that impacts Keller, Ryan, Wagner. E.g., This is likely why the call went out from UF to Insel to make sure Nemeroff would still be eligible for funding. To make sure he would be a taxpayer.
JAACAP/AACAP’s current stance remains to be seen.
That current stance should speak to how much trust physicians should place in the abstracts of work in JAACAP.
How can you have learnt from the mistakes that were made, if there were no mistakes? If there are no regrets. If you’re stating that everything was done with complete integrity and accuracy.
Is that the position AACAP takes, that given the same circumstances they would gladly do it all over again in an identical fashion?
GSK, to whatever extent they are to be believed, say the issues cannot and will not be ignored.
Perhaps JAACAP/AACAP does not differ with GSK on this point, has regrets about publishing the 2001 paper (including its abstract), and does not simply feel that the decision was a good one simply because the paper generated all sorts of useful discussion (admittedly, by that standard the paper was a home run).
But they certainly have not said anything to make that clear.
And perhaps their readership should keep that in mind the next time they see “J Am Acad Child Adolesc Psychiatry.” on an abstract and don’t have time to scrutinize the primary paper.
Try sending that message (NOT demanding retraction) to the 16 members of the AACAP council to keep in mind while they are “vetting” the issue.
The retraction gives them a straw man to respond to.
If you got some physicians to sign off on that kind of letter, one expressing concern about what the publishing of the 2001 paper/abstract says about JAACAP and asking what action they plan to take to reassure physicians that this does not reflect the expected level of scholarship in the abstracts they will encounter from JAACAP on pubmed in the future.
http://www.aacap.org/cs/root/…/understanding_how_aacap_works
If anyone could make that happen it would be you, 1BOM.
If not now when? If not you who?
There would probably also be no better time to ask than before this:
http://www.aacap.org/cs/AnnualMeeting/2012
How AACAP works says that there is an assembly of grassroots organizations that happens at that meeting. Give them an opportunity to address this.
In the face of a 3 billion dollar settlement from GSK where a JAACAP 2001 paper (that the senior editor at the time expresses no regrets about having published, with an abstract that describes effectiveness even though it was negative on every predetermined primary and secondary endpoint and that describes the safety issues in a way that can be judged on its own merits) was literally exhibit #2 in the trial.
I’ll sign off, but please consider it.
Well, Dr. Mickey, looks like Annonymous has been loading the artillery for YOU.
Will AACAP take more kindly to condemnation of the editorial standards of the J Am Acad Child Adolesc Psychiatry than a call for retraction of one paper?