LONDON [MarketWatch] – AstraZeneca … said Thursday that the European Commission has issued a positive decision for the approval of its Seroquel XR extended release tablets as an add-on treatment of major depressive episodes for patients who have sub-optimal response to antidepressant monotherapy. The company said the decision follows a recommendation by the Committee for Medicinal Products for Human Use in April. AstraZeneca said it will now move forward in obtaining local approvals, which is a 30-day process for the 17 member states that have signed up to a mutual recognition procedure.
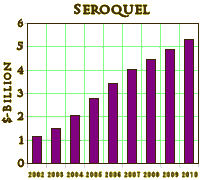 While there’s competition for the title, SEROQUEL® may be the paradigm for pharmaceutical marketing success in Psychiatric drugs. Introduced as an antipsychotic for the treatment of Schizophrenia in 1997, it was subsequently approved as an adjunct for the treatment of various aspects of bipolar disorder, then got a pediatric use approval, and now is looking to extend the patent period with SEROQUEL XR® as in the above. The potential side effect profile is ominous – Diabetes, weight gain, the full gamut of neuroleptic syndromes, and sudden death. Much of the success of the drug has to do with off-label uses as a tranquillizer in any number of conditions – notably PTSD [Questions loom over drug given to sleepless vets] where it is widely used for insomnia. The net result is that sales just keep on rising [$4.87 Billion last year].
While there’s competition for the title, SEROQUEL® may be the paradigm for pharmaceutical marketing success in Psychiatric drugs. Introduced as an antipsychotic for the treatment of Schizophrenia in 1997, it was subsequently approved as an adjunct for the treatment of various aspects of bipolar disorder, then got a pediatric use approval, and now is looking to extend the patent period with SEROQUEL XR® as in the above. The potential side effect profile is ominous – Diabetes, weight gain, the full gamut of neuroleptic syndromes, and sudden death. Much of the success of the drug has to do with off-label uses as a tranquillizer in any number of conditions – notably PTSD [Questions loom over drug given to sleepless vets] where it is widely used for insomnia. The net result is that sales just keep on rising [$4.87 Billion last year]. Other than aggressive marketing, why is it so widely prescribed? That’s actually a pretty good question that, as a clinician, I can’t answer. It’s a fair antipsychotic, though only about a third of patients will continue to take it long term. It apparently helps troubled people sleep. And it’s reported to help depressed people who haven’t responded to antidepressants. In my hands, it’s a mild "calmer downer" people take until they start gaining weight – then they stop. It fills no therapeutic niche that I can personally see. I’ve stopped prescribing it except in cases where people have been on it for a while and I don’t want to upset the apple cart [not many people]. But that’s just my experience [not in the current evidence based medicine craze bracket]. My guess is that it is widely prescribed by non-psychiatrists [who have heard about it in some Journal or CME presentation] to the sea of patients who report being "depressed" and didn’t respond to the SSRI du jour – that would be a very mixed bag of people ranging from the unemployed, through the unhappily married or unhappily unmarried, through the personality disordered, running into the depressed, and even some bipolar patients [who either have deep pockets or a willing prescription plan].
Other than aggressive marketing, why is it so widely prescribed? That’s actually a pretty good question that, as a clinician, I can’t answer. It’s a fair antipsychotic, though only about a third of patients will continue to take it long term. It apparently helps troubled people sleep. And it’s reported to help depressed people who haven’t responded to antidepressants. In my hands, it’s a mild "calmer downer" people take until they start gaining weight – then they stop. It fills no therapeutic niche that I can personally see. I’ve stopped prescribing it except in cases where people have been on it for a while and I don’t want to upset the apple cart [not many people]. But that’s just my experience [not in the current evidence based medicine craze bracket]. My guess is that it is widely prescribed by non-psychiatrists [who have heard about it in some Journal or CME presentation] to the sea of patients who report being "depressed" and didn’t respond to the SSRI du jour – that would be a very mixed bag of people ranging from the unemployed, through the unhappily married or unhappily unmarried, through the personality disordered, running into the depressed, and even some bipolar patients [who either have deep pockets or a willing prescription plan].
What does one do for the unhappy and the nervous people who visit their doctors in hope of help, particularly in an age when physicians are pushed to see people in increasingly brief aliquots of time? It’s a long story: Bromides, barbituates, benzodiazepams, SSRI’s – a history of symptomatic treatments, tranquillizers, whose dangers appear only after years of use. The problem with the widespread prescribing of Zyprexa, Abilify, Seroquel [the atypical antipsychotics] is that the side effects can be dire. But that’s a complaint that should be leveled at the prescribing physicians.
The complaint against drug companies is more specific, and AstraZeneca is a major offender. They have found a way to recommend the use of these drugs to physicians without acknowledging that it is advertisement. They hire Scientific Advisers and Speaker Bureaus of prominent physicians [KOL – Key Opinion Leaders] to give CME presentations [required Continuing Medical Education] and publish company financed research in free medical journals that appear in doctor’s mailboxes. When possible, this kind of research makes it into the higher level journals. It’s working for AstraZeneca to the tune of $4+ Billion/year – never mind the suits they settle for small pieces of their profit. Now their patent is running out, they’re trying to extend it by getting approval for other diagnoses or other dosages [as above]. What’s the charge? They’re cheating, and the ultimate losers are patients who suffer the side effects and the doctors who think they’re practicing good medicine based on fallacious or filtered information.
 The real tragedy is that if you asked me where one should go to get accurate and safe recommendations about psychoactive medications right now, I’m not sure I’d be able to answer the question. My solution is to use older medications I have lots of experience with and shy away from almost all newer medications except in very circumscribed situations. And as a rule of thumb, I respond negatively to the rare person who asks, "Is ____ right for me?" I try not to scream, and usually jokingly say something like "I’m going to have to put you on television commercial restriction" or "Listen to that ‘what it can do to you’ part they mumble at the end again." My solution? Suspend the medical license of any physician who makes a CME presentation and who receives payment from a pharmaceutical company. CME presentation is not a right, it’s a privilege for the pure of heart [and purse]. Otherwise, eliminate the requirement for Continuing Medical Education altogether. It’s sad to see pharmaceutical manufacturers as the enemy of our patients, but that’s just the way they played the game…
The real tragedy is that if you asked me where one should go to get accurate and safe recommendations about psychoactive medications right now, I’m not sure I’d be able to answer the question. My solution is to use older medications I have lots of experience with and shy away from almost all newer medications except in very circumscribed situations. And as a rule of thumb, I respond negatively to the rare person who asks, "Is ____ right for me?" I try not to scream, and usually jokingly say something like "I’m going to have to put you on television commercial restriction" or "Listen to that ‘what it can do to you’ part they mumble at the end again." My solution? Suspend the medical license of any physician who makes a CME presentation and who receives payment from a pharmaceutical company. CME presentation is not a right, it’s a privilege for the pure of heart [and purse]. Otherwise, eliminate the requirement for Continuing Medical Education altogether. It’s sad to see pharmaceutical manufacturers as the enemy of our patients, but that’s just the way they played the game…
Great post, I hope your colleagues read your blog.