 So we had a New York Times front page ad for $eroquel XR that says "Adding SEROQUEL XR for depression may help." Down at the bottom it says "Roll-over for MORE Important Safety Information" in small print. As mentioned before in this blog, $eroquel is an Atypical Antipsychotic originally approved by the FDA for the treatment of Schizophrenia [1997]. Since then, it has been approved for other things but it has been at the center of innumerable suits for false advertising, and harming patients. For example, the manufacturer settled a suit for $520 M, but since they make $4-5 B from the drug yearly, what’s half a billion in the scope of things? Truth be told, $eroquel is a paradigmatic example of the excesses of PHARMA. Every piece of this ad is carefully and deceptively phrased:
So we had a New York Times front page ad for $eroquel XR that says "Adding SEROQUEL XR for depression may help." Down at the bottom it says "Roll-over for MORE Important Safety Information" in small print. As mentioned before in this blog, $eroquel is an Atypical Antipsychotic originally approved by the FDA for the treatment of Schizophrenia [1997]. Since then, it has been approved for other things but it has been at the center of innumerable suits for false advertising, and harming patients. For example, the manufacturer settled a suit for $520 M, but since they make $4-5 B from the drug yearly, what’s half a billion in the scope of things? Truth be told, $eroquel is a paradigmatic example of the excesses of PHARMA. Every piece of this ad is carefully and deceptively phrased:"SEROQUEL" : $eroquel is an Atypical Antipsychotic approved for the treatment of Schizophrenia because these drugs may have less propensity to have neurological consequences [Tardive Dyskinesia]. It is less effective as an antipsychotic than the older drugs.
"XR" : Adding a time release pill is a way of having something new to sell. It adds little, if anything to the therapeutic effectiveness of the drug.
"DEPRESSION" : In the lay use of the term, depression can mean many things. ranging from "unhappiness" to debilitating illness. Major Depressive Disorder is a small percentage of the people who report being depressed.
The pharmaceutical company AstraZeneca said Thursday that it had reached a $520 million agreement to settle two federal investigations and two whistle-blower lawsuits over the sale and marketing of its blockbuster psychiatric drug Seroquel. One of the investigations related to “selected physicians who participated in clinical trials involving Seroquel,” AstraZeneca disclosed in a government filing. The other case related to off-label promotion of the drug.
As a result of aggressive marketing, Seroquel has been increasingly used for children and elderly people for indications not approved by the Food and Drug Administration. Doctors are permitted to prescribe any approved drug for off-label uses. Seroquel was the top-selling antipsychotic drug in America. It had $17 billion in sales in the United States since 2004, according to IMS Health, a research firm…
Mr. Blizzard said he did not know what clinical trials were part of the inquiry. But in one trial, known as Study 15, he noted, an e-mail message showed a company official saying “a great ‘smoke and mirrors’ job’ ” had been done on a “buried” study in 1997, the year the F.D.A. approved Seroquel…
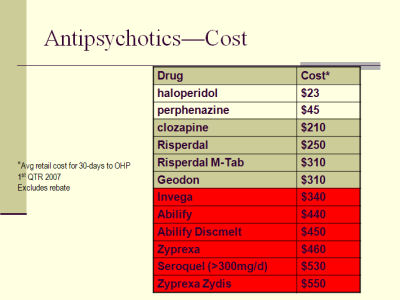
 While I would personally see this ad as false advertising, they’ve stayed inside the letter of the law [barely]. This ad is driven by this fact – "Seroquel was the top-selling antipsychotic drug in America. It had $17 billion in sales in the United States since 2004." Since it is potentially toxic and probably only really useful in cases where the person taking it is psychotically depressed, it’s the kind of thing you might try if you’re treating someone like Virginia Woolf or William Styron [Darkness Visible] – people with life-threatening Depressive Illness.
While I would personally see this ad as false advertising, they’ve stayed inside the letter of the law [barely]. This ad is driven by this fact – "Seroquel was the top-selling antipsychotic drug in America. It had $17 billion in sales in the United States since 2004." Since it is potentially toxic and probably only really useful in cases where the person taking it is psychotically depressed, it’s the kind of thing you might try if you’re treating someone like Virginia Woolf or William Styron [Darkness Visible] – people with life-threatening Depressive Illness.But this ad is aimed at a different group – the people who might read ads in the New York Times. And the point is to get someone who is depressed who hasn’t responded to an SSRI to mention it to their Primary Care Physician – neither doctor nor patient having read Questions loom over drug given to sleepless vets, AstraZeneca gives investors a boost, AstraZeneca Said to Pay $55 Million Over Seroquel, AstraZeneca Pays Millions to Settle Seroquel Cases, or the countless other reports about Seroquel.
This $eroquel is the shit that Charles Nemeroff took money to push for patients with non-psychotic depression. See Dr. Carroll’s exposé here.
http://hcrenewal.blogspot.com/2009/10/nemeroff-seroquel-and-accme.html
This was a big deal tele-presentation brokered by one of the parasite CME companies. Nemeroff’s program was so bad that it was sanctioned by the Accreditation Council for Continuing Medical Education. The company was ordered to pull the program from its website.
We can only hope that the University of Miami now has the toxic Nemeroff on a short leash to prevent him from peddling this shit. That will be a full time job for them, as Emory discovered.
These ads are everywhere from FOX news to Psychology Today web sites…Truly disgusting that we have a culture that is willing to promote profit over health consistently.
AstraZeneca is a Corporation operating under a CIA corporate Integrity Agreement for no other than misrepresenting the very drug they are running these ads for – ethical behavior & agreements obviously mean nothing.
Good work here.
The deceit of AstraZeneca takes your breath away. Like here, for example, in the safety information: Antidepressants have increased the risk of suicidal thoughts and actions in some children, teenagers and young adults. Patients of all ages starting treatment should be watched closely for worsening of depression, suicidal thoughts or actions, unusual changes in behavior, agitation and irritability. Patients, families and caregivers should pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts or feelings. This is very important when an antidepressant medication is started or when the dose is changed…. Notice the sly labeling of Seroquel as an antidepressant drug!
Makes you wonder why the FDA allows this kind of stealth marketing to be slipped into the warning notice, doesn’t it?
Thanks for the comments. I’m amazed at their boldness given their losses in the courts. I guess that the “cost of doing business” argument is correct. They need a real hit from a class action suit that would level the playing field.
Anyone would be a fool/mislead to believe this was about treatment or even about medicine. It’s about marketing and profits….According to this article, AstraZeneca did it best last year.
http://www.bioportfolio.com/news/article/366222/Mm-m-All-stars-Large-Pharma-Marketing-Team-Of-The-Year-Seroquel.html
[…] investigation of this drug and a suspension of further indications instead of this campaign [informed consent?…]. Now, as AstraZeneca rolls out its "Still Trying to Get Ahead of Your Depression" […]