Corcept did indeed go public in the Spring of 2004, selling shares worth over $50 M. In September of 2005, the Stanford group published a third Mifepristone study online [in print in on March 2006]. At the time, Dr. Charles Nemeroff was the editor of
Neuropsychopharmacology [the Journal that published the study]. His editorship ended abruptly not long thereafter. In April 2006, he and eight other authors published a "perspective" article in that Journal [
VNS Therapy in Treatment-Resistant Depression: Clinical Evidence and Putative Neurobiological Mechanisms. Neuropsychopharmacology (2006)
31, 1345–1355]. And then:
The editor of a medical journal has resigned after drawing sharp criticism for failing to disclose his financial ties to a medical-device manufacturer after publishing an paper that endorsed one of its products. The editor, Charles B. Nemeroff, chairman of Emory University’s department of psychiatry and behavioral sciences, was also a co-author of the paper, which said the device was an effective treatment for depression. The medical society that publishes the journal, Neuropsychopharmacology, attributed his resignation, in part, to bad publicity over the paper.
All of that news comes from today’s Wall Street Journal, which last month published a long article about the medical journal’s failure to note that Dr. Nemeroff and seven of his eight academic co-authors had financial ties to the manufacturer, Cyberonics Inc. A ninth co-author was an employee of the company. The medical journal subsequently published a correction noting those relationships.
I guess Dr. Nemeroff forgot what he’d
written in 2003 in his response to Drs. Carroll and Rubin: "
Going forward, we intend to provide all financial disclosure information, even if it is not requested by the journal editor."
So back to the third Mifepristone study:
|
|
|
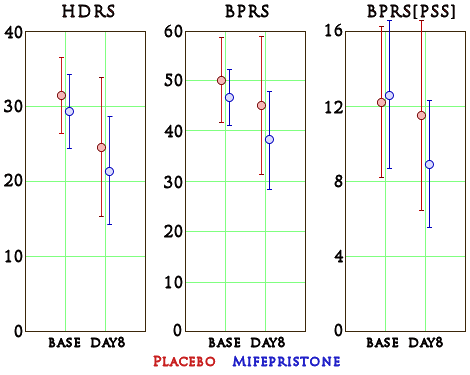
Psychotic major depression [PMD] is found to be a relatively common psychiatric condition that affects up to nearly 20% of patients with major depression. Previous studies by our group have shown rapid reversal of psychotic symptoms in some PMD patients treated with mifepristone, in addition to restoring a more normal afternoon cortisol release. The rationale for treating patients with PMD with a glucocorticosteroid receptor antagonist is further discussed. In total, 30 patients with PMD were treated with either 600 mg/day mifepristone or placebo for 8 days in a randomized double-blind manner. The Hamilton Depression Rating Scale [HDRS] and the Brief Psychiatric Rating Scale [BPRS] were administered at baseline and again after 8 days of treatment. Cortisol and ACTH were measured hourly from 1800 to 0900 at baseline and after 8 days of treatment. Significantly, more patients in the mifepristone group [seven of 15] showed a 50% or greater decline on the BPRS positive symptom subscale, an index of psychotic symptoms, as compared to the placebo group [two of 15]. Patients who received mifepristone had lower HDRS and BPRS scores at study completion compared to those who received placebo, but these differences were not statistically significant… These results suggest that short-term use of mifepristone may be effective in the treatment of PMD and may re-regulate the HPA axis. Additional blinded studies are warranted.
|
This was an NIMH funded study – a double blind comparison of Placebo and Mifepristone [n=30] done by Stanford. The outcome measures were the same scales as before [Hamilton Depression Rating Scale, Brief Psychiatric Rating Scale, and the BPRS Positive Symptom Subscale]. Here are the raw results [mean with 1 SD above and below]:
The
Analysis of Variance table showed no significance except time, though the BPRS[PSS] scores were reported in an unusual way:
Using repeated-measures ANOVA, we found no main effect of medication for the BPRS positive symptoms subscale (F(1,28)=0.765, NS), BPRS total score (F(1,28) =2.710, p<0.12), or HDRS total score (F(1,28)=1.937, NS). There was a significant main effect of time for the BPRS PSS (F(1,28)=8.062, p<0.01), BPRS total score (F(1,28)=12.271, p<0.01), and HDRS total score (F(1,28)=23.150, p<0.001), such that significant clinical improvement was observed in both the mifepristone and placebo groups at Day 8. The medication-by-time interaction in BPRS positive symptoms (F(1,28)=3.916, p=0.058) was nearly significant, while the interactions with medications were not significant for BPRS total score (F(1,28)=0.937, NS) or HDRS total score (F(1,28)=0.118, NS).
Since the point of statistical analysis is to produce yes/no results, the comment "The medication-by-time interaction in BPRS positive symptoms … was
nearly significant" is, at best, odd [like
nearly legal or
nearly pregnant would sound]. My own reading of the ANOVA table was more "close, but no cigar" and "time heals all wounds" rather than anything about the drug Mifepristone. Using a panoply of other statistical tests however, they felt that the BPRS[PSS] result was significant. I’ll refer you to the
online article and the
reanalysis for your own conclusions.
Drs. Carroll and Rubin responded again, and this time their analysis was published along the the authors’ response [Keller and Schatzberg]. Their critique is extensive and should be read
in toto. Among other things, they address the
chase for significance. In essence, if you use multiple statistical tests, you should correct the probabilities for the number of tests used. This is a way of testing the
significance of significance. A tongue-twister, but a standard correction that this article lacked, and it ablates the reported significance. Their conclusions [
Is Mifepristone Useful in Psychotic Depression? Neuropsychopharmacology (2006) 31, 2793-2794.]:
… In summary, the authors have made an invalid claim of efficacy for mifepristone in PMD, based on inadequate statistical analyses. The authors have made an invalid claim of clinical utility based on an inadequate measure of that construct. The population studied has dubious generalizability to clinical practice. And the authors have made an unjustified claim of beneficial neuroendocrine change based on weak experimental design and on a fundamental confusion about re-regulation of the HPA axis in PMD.
We must reiterate that this was a pilot study, not a definitive study designed to answer all possible questions about mifepristone. What would constitute a definitive study to investigate the mechanisms of drug action or to investigate the long-term effects of the drug would be very different…
It is clear that only larger-scale trials will allow us to truly determine whether the medication is useful and whether it improves both psychosis and depression…
Overall, the purpose and the conclusions of our study appear to have been ignored by Rubin and Carroll, and we are concerned that their letter will confuse others. Ongoing trials hopefully will help clarify the range of efficacy of GR antagonists in patients with this severe disorder.
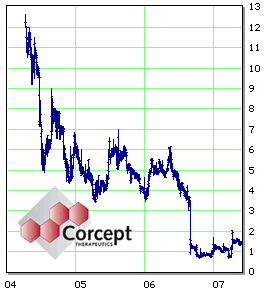
As 2006 progressed, the Phase III Clinical Trials were scheduled to be reported. Fall must’ve gotten pretty depressing around the Menlo Park offices of
Corcept. Not only did Scientific Board member, Dr. Nemeroff, have to resign his
Neuropsychopharmacology editorship in disgrace, but also the first two Phase III Clinical Trials of Mifepristone in Psychotic Depression were reported in
August and
September and failed to meet their goals for significance. So there was one outcome measure that needed no probability analysis to understand – the value of
Corcept‘s Stock:
Let me remind you that the reason for this series is not that I have any primary interest in Mifepristone itself. The patients I’ve seen with Psychotic Depression are gravely ill and our current treatment armamentarium for them is weak. I’d be glad if there were a safe and robust treatment for those patients. They do, indeed, suffer. The thrust of these posts is about the extreme Conflict of Interest in this story – the actual founding of a pharmaceutical company by prominent Academic Psychiatrists who then use their own NIMH funded studies and their positions as "key opinion leaders" in the field to promote their product in our Journals, without acknowledging their financial interests [promotions, I might add, that I think way overstate their data]. In my opinion, this would stink if Mifepristone turned out to be "the best thing since white bread" [which it isn’t], but it smells even worse when it’s rife with deceit about the drug’s effectiveness. As we will see, others in higher places shared this opinion…


Mickey — I just want to say that I really appreciate your researching and then writing these posts. I would know only the most superficial aspect of this debacle if it were not for your posts.