I skipped over some things because my real interest is the corruption of Academic medicine, not so much in the spin and grin of a start-up pharmaceutical company. By the end of 2006 with two failed clinical Phase III clinical trials, Corcept was in trouble. They published a study that I think had been done several years earlier [mentioned as early as 2003], this time without Schatzberg as an author:
|
Biological Psychiatry by DeBattista C, Belanoff J, Glass S, Khan A, Horne RL, Blasey C, Carpenter LL, and Alva G. Corcept Therapeutics, Menlo Park, California December 2006 |
|
BACKGROUND: Abnormalities in the hypothalamic pituitary adrenal axis have been implicated in the pathophysiology of psychotic major depression [PMD]. Recent studies have suggested that the antiglucocorticoid, mifepristone might have a role in the treatment of PMD. The current study tested the efficacy of mifepristone treatment of the psychotic symptoms of PMD.
METHODS: 221 patients, aged 19 to 75 years, who met DSM-IV and SCID criteria for PMD and were not receiving antidepressants or antipsychotics, participated in a double blind, randomized, placebo controlled study. Patients were randomly assigned to either 7 days of mifepristone [n = 105] or placebo [n = 116] followed by 21 days of usual treatment.
RESULTS: Patients treated with mifepristone were significantly more likely to achieve response, defined as a 30% reduction in the Brief Psychiatric Rating Scale [BPRS]. In addition, mifepristone treated patients were significantly more likely to achieve a 50% reduction in the BPRS Positive Symptom Scale [PSS]. No significant differences were observed on measures of depression.
CONCLUSION: A seven day course of mifepristone followed by usual treatment appears to be effective and well tolerated in the treatment of psychosis in PMD. This study suggests that the antiglucocorticoid, mifepristone, might represent an alternative to traditional treatments of psychosis in psychotic depression.
|
Candidly, I wouldn’t have put my name on it either. It was a double-blind, placebo-controlled study done in 29 centers. The subjects took either Mifepristone or placebo for 7 days, then were treated for 21 days with the treatment du jour by their treating physicians [anti-depressants, antipsychotics, ECT, etc.] then retested. The outcome parameters were the usual [HAM-D, BPRS, BPRS[PSS]]. Response was reported as non-response, response, rapid and sustained response based on achieving pre-defined % fall in scores. 30% had missing data filled in by some mathematical mojo. The response category included responders at 7 or 28 days [couldn’t tell which]. The rapid and sustained group responded at both times. The only significance was if and only if they mathematically inserted missing data. It was not significant for the data they actually observed. I have no idea if it would’ve achieved significance if the raw scores were analyzed. I really doubt it because if that were true, they wouldn’t have hidden their primary data. I was taught to ignore studies with this much data manipulation and obfuscation [for a very good reason!]. I’m frankly surprised that this muddy study was even published.
Then in March of 2007, the third Phase III trial was released:
Corcept Therapeutics Announces Phase 3 Study Evaluating CORLUX(R) for Psychotic Major Depression Misses Primary Endpoint
Marketwire
March 20, 2007
Corcept Therapeutics Incorporated today announced that Study 06, the last of three Phase 3 trials evaluating CORLUX for treating the psychotic features of Psychotic Major Depression (PMD), did not achieve statistical significance with respect to its primary endpoint. However, there was a statistically significant correlation between plasma levels and clinical outcome achieved during treatment. Further, the company reported that the incidence of serious adverse events did not differ between placebo and any of the three CORLUX dose groups. Patients whose plasma levels rose above a predetermined threshold statistically separated from both those whose plasma levels were below the threshold and those patients who received placebo. This confirmed a similar finding in Study 07, another Phase 3 trial testing CORLUX for PMD completed in 2006. "While we are disappointed that the trial did not meet the primary endpoint, we are particularly encouraged to have met the important predefined threshold concentration endpoint with statistical significance," said Joseph K. Belanoff, M.D., Corcept’s Chief Executive Officer. "This study confirms our previous observation that at higher plasma levels the drug candidate is able to demonstrate desired clinical effects. In particular, those patients in Study 06 who achieved a predetermined level of 1661 nanograms of CORLUX per milliliter of plasma separated from the placebo group with statistical significance"…
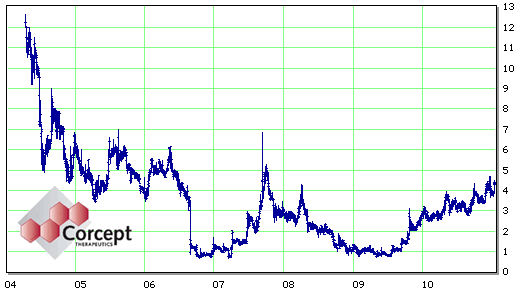
At this point, Dr. Schatzberg was out of the game and the stock value was in the tank. But hope springs eternal at Corcept. They obtained "orphan" status [a 7 years exclusivity status] by agreeing to study Mifepristone in Cushing’s Disease [an infrequent disease where treatment is needed] and initiated Phase III trials. They also started another trial for Psychotic Depression [higher dose?]. At this point [right now] there’s great excitement because the Phase III Clinical Trial for Cushing’s is a success. I expect they envision approval for Cushing’s [rare], extending it to Psychotic Depression [not common], then aiming to creep into "Treatment Resistant Depression" [common]. Like I said, hope springs eternal at Corcept.
Mickey, I enjoyed reading your entire series on mifepristone.
When I was a study coordinator at UT Southwestern Medical Center in 2005, I assisted briefly with the site initiation for Study 06 (ClinicalTrials.gov Identifier: NCT00128479). Madhukar Trivedi was the site investigator and a paid consultant to Corcept Therapeutics.
According to the information posted on ClinicalTrials.gov, the multi-site study enrollment was completed in February 2007. Twenty-one of 44 study sites were terminated and one site was suspended during the study, by my count. See
http://tinyurl.com/4zwo6bh
I am curious about the reasons for the site suspension and terminations, and the justification why Study 06 was not an inpatient study due to the high-risk population of subjects. My specific concerns include 1) the safety of these subjects during the washout period; 2) the use of placebo in a group of high-risk subjects; 3) the suicide risk associated with titration of the antidepressants, especially in a group of subjects with psychotic features; and 4) safety concerns regarding possible Addisonian episodes.