Everyone knew what was wanted: a potent antipsychotic drug that did not have extrapyramidal symptoms. Clozapine had filled the bill, but brought along a couple of new possibilities – fatal agranulocytosis, weight gain, and glucose intolerance. So the race was on to find a clozapine-like variant without these deal-breakers. Zeneca hoped they had it in Seroquel. In the F.D.A. approval trials, there was ample evidence that they weren’t quite there. It was an antipsychotic, but it was lower potency than they wanted – no better that Thorazine [Trial 0007] and inferior to Haldol [Trial 0014 and Trial 0015]. Extrapyramidal problems were diminished and it didn’t kill the bone marrow like Clozapine, but hope though they might, the longer they gave it, the more weight gain they saw. If they were concerned about Diabetes, they kept it to themselves. And they dealt with the downsides of their drug by hiding them. It wasn’t simply a case of putting their "best foot forward," it was more like the 40’s song "accentuate the positive, eliminate the negative, latch on to the affirmative, and don’t mess with Mr. In-between." And when Seroquel came onto market, it was Mr. In-between in a line-up of competing Atypical Antipsychotics.

In their Strategic Plan [1997-2001] at launch time, Zeneca seemed worried [a few highlights]:

They didn’t have anything to separate them from the pack. They had apparently been counting on Trial 0015 to give them a relapse prevention selling point, but that trial bombed and they buried it from sight. They were afraid to try it again for fear of failure. So, among other things, they decided to target patient advocacy groups and invest in short-term clinical trials that might broaden their indications – a strategy they pursued with a vengeance. A new Seroquel Strategic Plan in 2000 was more aggressive:
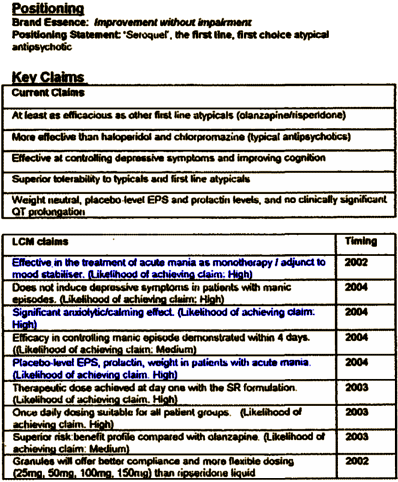
Most of the "Key Claims" would ultimately turn out to be, at best, overstated. In the "LCM Claims," they set out an action plan that they actually followed. The plan [items 1, 3, & 5] marks the beginning of their indication creep – adding other indications for Seroquel to expand their market. They included Anxiety and Mania, but they were just getting warmed up for what was to come. There was another part to this plan – "Key Success Factors:"
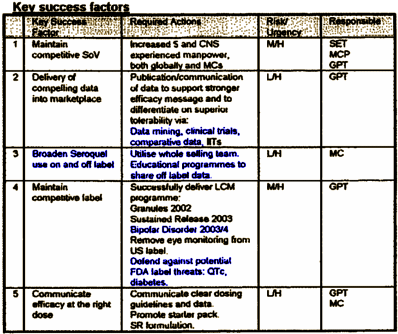
I expect that many of us would be put off by any strategic marketing plan for a pharmaceutical medication, but this one maybe more than most. In number 2., notice the terms "data mining" and clinical trials [317 in clinicaltrials.gov since 2004, 155 financed by AstraZeneca]. Number 3., "off label" includes "educational programs to share off-label data" [which happens to be against the F.D.A. guidelines]. In practice, this lead to C.M.E. Programs and Speaker Bureau presentations to disseminate these "off label" recommendations. In number 4., they plan more indication creep to Bipolar Disorder by 2003-2004. And they’re steeling themselves for F.D.A. fights about Diabetes [You just don’t plan to defend yourself against future accusations that your drug causes Diabetes unless your drug maybe causes Diabetes].
Now they had a strategy: keep up with the well-tolerated, weight-neutral, EPS-less, potent antipsychotic line, but begin a campaign to expand the market outside of Schizophrenia into Anxiety, Mania, Bipolar Disorders [can Depression be far behind?]. As they headed for the "off label" market, they invested in Clinical Trials of Seroquel in these other diagnostic arenas, and pushed "educational programmes to share off label data."
Weight-Neutral continued to be an essential part of their strategy. The subpoenaed internal documents open the window to the back story of their deliberations about weight. They had developed a single case brochure [I’ve reformatted it to make it fit]:

The long-term effect of quetiapine (Seroquel™)monotherapy on weight in patients with schizophrenia
International Journal of Psychiatry in Clinical Practice Vol. 4: 287 – 291, 2000.
by M. BRECHER, I.W. RAK, K. MELVIN and A.M. JONES
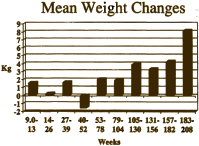
… Minimal overall weight change was observed over 18 months of treatment with quetiapine. The mean weight change from baseline was: 1.58 kg after 9-13 weeks (n=170); 0.26 kg after 14-26 weeks (n=165); 1.66 kg after 27-39 weeks (n=134); 1.53 kg after 40-52 weeks (n=41); and 1.94 kg after 53-78 weeks (n=146)… Results of the present analysis show that, in clinical studies where no other antipsychotic medications were permitted during the OLE phase of treatment, quetiapine was associated with only minimal changes in weight in the short term [8 weeks], and with an overall neutral effect on weight with long-term treatment. By comparison, an increase of approximately 12 kg has been reported after 12 months’ treatment with olanzapine 12.5 ± 17.5 mg/day…
 in their Core Data Sheet, but after the F.D.A. query about Diabetes, they began to discuss removing the word "limited."
in their Core Data Sheet, but after the F.D.A. query about Diabetes, they began to discuss removing the word "limited."Then somebody at AstraZeneca noticed the obvious – that they had more than just 18 months of data on this group of subjects that had been used for the published paper [see the graph on the right that goes with the email below]. The authors had simply cut off the part they didn’t like [18 months to four years].

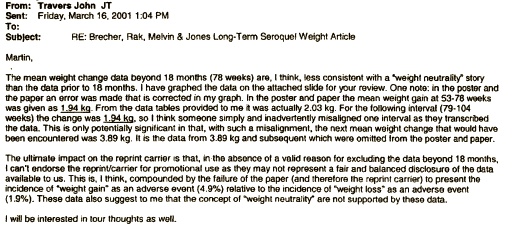
Great job Mickey. Where did you find the Travers email at the end?
at the psych rights site, jim gottsteins…the email
When and if the mood strikes you, would you please consider posting a bit about your views on the process by which a prescriber (psychiatrist or physician prescribing psychotropics) and patient optimally decide on medication(s) as therapy?
I appreciate the thought and evidence you are bringing to the interface (more like face palm) between psychiatry, pharma and patient treatment. It’s painful reading, and I’m empathetic about the distress it’s brought you.
Mickey, we are all in your debt for this research and synthesis of the information about Seroquel. It is a case study for the entire industry.
“On the other hand, clinical studies that extend the indications for Seroquel can directly impact sales…. Funding of clinical studies will therefore come first.†This quote from the Jeffrey Goldstein memorandum in 1997 is a pure expression of the scientific priorities inside Pharma. It captures the mindset that led me to coin the term ‘experimercial’ in 2004 for commercially strategic clinical trials that are designed to promote a drug in a market niche.
In the conduct of experimercials, human subjects are not patients, they are commodities, as you pointed out. Dan Markingson allegedly was being used as such a commodity when he died at The University of Minnesota in the AZ funded comparative trial of Seroquel versus other atypical antipsychotic drugs (see here ).
Here is the url: http://tinyurl.com/39a5btb
Two pictures are worth a thousand words!
http://pharmagossip.blogspot.com/2011/02/seroquel-how-did-astrazeneca-make-it.html
Glad to see Bernard Carroll here. Esp when I have to tolerate this:
http://twitter.com/carlatblog/status/40128477919318016
Dr Daniel Carlat calling this ‘seroquel bashing’. He could stand to learn a thing or 2 from this blog, that’s for sure.