 Joe: I was looking over some of those trials we buried. Seroquel is kind of a lightweight in spite of all our hype, isn’t it?
Joe: I was looking over some of those trials we buried. Seroquel is kind of a lightweight in spite of all our hype, isn’t it?Jim: Yeah. I mean it’s well tolerated except for that pesky weight/diabetes thing, and it seems to calm people down, puts them to sleep. But it’s a softy as antipsychotics go. It’s a shame. Getting it on the market was hard work and cost Zeneca a bundle.
Joe: I’ve been wondering… What if, instead of seeing it as a wimpy antipsychotic, we took advantage of that and called it a strong anti-anxiety drug, or a novel anti-depressant, or something like that? What’s going to fill the benzodiazepine niche anyway?
Jim: You mean make a silk purse out of this sow’s ear? You’re a real dreamer, Joe!
While I have no evidence that such a conversation ever took place in reality, it might as well have, because that’s what seems to have happened [even though Seroquel stayed in the battle of the Atypical Antipsychotics in treating Schizophrenia]. Whatever the thoughts behind the curtain, the idea of indication creep was there from the outset [Jeffrey Goldstein, November 1997]:
Looking at all these clinical trials graphs, I got to wondering if one could graph indication creep [a case of graph envy?]. I don’t think it’ll get me an NIMH grant, but I just counted the articles in pubmed that contained "QUETIAPINE" and had various words in the [TITLE]. It’s displayed with the F.D.A. approval dates in the table. …something of a story:
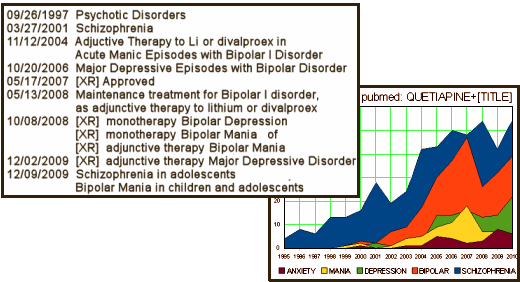
Each of the approvals is supported by Clinical Trials submitted to the F.D.A., [connecting the actual trial with the approval is difficult as there is very little information on the F.D.A. site for supplemental approvals]. But most of the studies are published. I’ve picked one of the recent ones as an example because there’s greater likelihood of full disclosure and it is available full text on the Internet. This one is Seroquel XR as the sole treatment in MDD [Seroquel was not approved as monotherapy in MDD on the grounds that the side effects outweigh the gain]:
Results of a Double-Blind, Randomized, Placebo-Controlled Study
by Richard Weisler, J. Mark Joyce, Lora McGill, Arthur Lazarus, Johan Szamosi, and Hans Eriksson
CNS Spectrums 2009;14(6):299-313
Introduction: Once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy was evaluated in major depressive disorder (MDD).Method: This was an 8-week (6-week randomized-phase; 2-week drug-discontinuation/tapering phase), double-blind, parallel-group, placebo-controlled study. The primary outcome measure was Montgomery-Åsberg Depression Rating Scale (MADRS) total score randomization-to-Week 6 change. Other assessments included the Hamilton Rating Scale for Depression, the Hamilton Rating Scale for Anxiety, and adverse events (AEs).Results: 723 patients were randomized: 182, 178, 179, and 184 to quetiapine XR 50, 150, 300 mg/day, and placebo, respectively. At Week 6, significant reductions occurred in MADRS score with quetiapine XR 50 mg/day (–13.56; P<.05), 150 mg/day (–14.50; P<.01) and 300 mg/day (–14.18; P<.01) versus placebo (–11.07); at Day 4, reductions for quetiapine XR (titrated to 50 or 150 mg/day according to dose group) versus placebo (–2.9) were: –4.7 (P<.01), –5.2 (P<.001), and –5.1 (P<.001), respectively. At endpoint, MADRS response (≥50% reduction in score) was 42.7% (P<.01), 51.2% (P<.001), and 44.9% (P≤.001) for quetiapine XR 50, 150, and 300 mg/day, respectively; 30.3% for placebo. Overall, quetiapine XR 150 mg/day provided consistently more positive secondary efficacy results than 50 mg/day and 300 mg/day versus placebo. The most common AEs in quetiapine XR-treated patients were dry mouth, sedation, somnolence, headache, and dizziness.Conclusion: In patients with MDD, quetiapine XR monotherapy (50/150/300 mg/day) is effective in reducing depressive symptoms, with improvement from Day 4 onwards. Safety and tolerability were consistent with the known profile of quetiapine.
Faculty Affiliations: Dr. Weisler is Adjunct Professor of Psychiatry in the Department of Psychiatry at the University of North Carolina, Chapel Hill, and Adjunct Associate Professor of Psychiatry at Duke University, in Durham, North Carolina. Dr. Joyce is Principal Investigator at CNS Healthcare in Jacksonville, FL. and Dr. McGill is Lead Principal Investigator at CNS Healthcare in Memphis. Dr. Lazarus, Mr. Szamosi, and Dr. Eriksson are employees of AstraZeneca.Funding/Support: The study (Moonstone; D1448C00001) was funded by AstraZeneca. The study was registered at ClinicalTrials.gov (identifier number NCT00320268).Acknowledgments: The authors would like to thank Jocelyn Woodcock, MPhil, from Complete Medical Communications, who provided medical writing support funded by AstraZeneca…
They are fairly monotonous double-blind placebo-controlled 6 week trials using a variety of rating scales. The lead author is usually academically affiliated and a consultant of some sort for a number of Pharmaceutical Companies [20], on a number of speaker’s bureaus [21], and a recipient grants from Pharmaceutical Companies [43]. There may be authors from the Clinical Trials Industry [CNS Healthcare]. And there are always several authors from AstraZeneca. The studies are funded by AstraZeneca, as is the contract medical writer [Complete Medical Communications]. This formula appears to be the standard fare:
-
an academically affiliated, AstraZenica affiliated lead author
-
a Clinical Trials Company that organizes and conducts the study
-
a Medical Writing Company that… well does something
-
AstraZeneca staff in a variety of capacities
-
AstraZeneca funding for the project
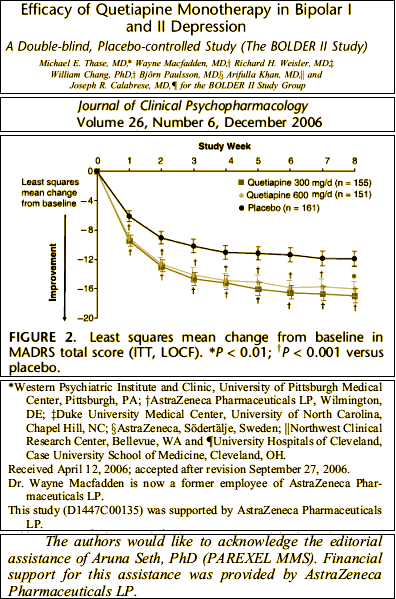
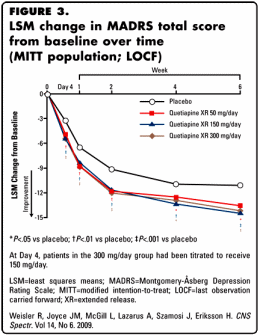 Looking over a number of these clinical trials, I found them annoying. Using this one as an example, the primary outcome scale was the Montgomery-Åsberg Depression Rating Scale [MADRS]. There’s obviously a difference between placebo and the medication [though the dose doesn’t seem to matter very much]. But there’s a lot of number manipulation involved in the graph. There were many drop-outs [~50%], but unlike the studies in the initial approval, we can’t see them. And the "modified intention-to-treat [MITT] [all patients assigned to randomized treatment who took study medication and who had a MADRS assessment at randomization and ≥1 valid MADRS assessment after randomization]" is a mouthful and a half. Then they used that LOCF [last observation carried forward] method to fill in for missing data. Put all that together and there’s no way to see the primary data. Just looking at the graph itself, it looks like just being in their study is really good for Major Depressive Disorder, and that adding a bit of Seroquel [in any dose] gives you a slight boost. We know from looking at these graphs before that if we’re given the full data, we can evaluate them more precisely, but the information just isn’t here. I don’t doubt that there is a statistical difference, but I question if what I see here is relevant – and there’s absolutely no way to find the answer. They included the more familiar Hamilton Depression Scale [HAM-D] values as a secondary outcome measure:
Looking over a number of these clinical trials, I found them annoying. Using this one as an example, the primary outcome scale was the Montgomery-Åsberg Depression Rating Scale [MADRS]. There’s obviously a difference between placebo and the medication [though the dose doesn’t seem to matter very much]. But there’s a lot of number manipulation involved in the graph. There were many drop-outs [~50%], but unlike the studies in the initial approval, we can’t see them. And the "modified intention-to-treat [MITT] [all patients assigned to randomized treatment who took study medication and who had a MADRS assessment at randomization and ≥1 valid MADRS assessment after randomization]" is a mouthful and a half. Then they used that LOCF [last observation carried forward] method to fill in for missing data. Put all that together and there’s no way to see the primary data. Just looking at the graph itself, it looks like just being in their study is really good for Major Depressive Disorder, and that adding a bit of Seroquel [in any dose] gives you a slight boost. We know from looking at these graphs before that if we’re given the full data, we can evaluate them more precisely, but the information just isn’t here. I don’t doubt that there is a statistical difference, but I question if what I see here is relevant – and there’s absolutely no way to find the answer. They included the more familiar Hamilton Depression Scale [HAM-D] values as a secondary outcome measure:Underwhelming. I’m a reasonably educated Psychiatrist, sort of a newbie to the modern ways of the Clinical Trials world but not to scientific medical information in general, and I can’t tell from what I see here if Seroquel XR helps people with Major Depressive Disorder in a meaningful way or not. My guess is not, but I can’t even say that for sure. I didn’t pick this to be an example of a bad study [though it seems like it is when I look at it closely]. I picked it to show the boiler-plate formula AstraZeneca used to creep through the DSM IV collecting indications for Seroquel. There are others with more convincing data, but the format remains the same – something of a data-factory.
While it was important to get the approvals so they could advertise the indications legitimately, it was also important to get the articles published to get their data out even before the approvals. But indication creep wasn’t just happening at AstraZeneca. It was going around the Atypical world:
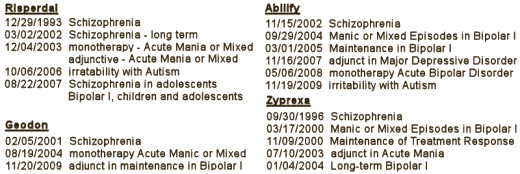
So indication creep alone can’t explain AstraZeneca‘s success story by itself, even though they excelled at it. There had to be some other factors involved. Next we’ll take a peek at off-label promotion.
UPDATE: Another example with better results [essential elements only included]:
Look familiar?…


The name of the game is to promote a drug like Seroquel as a broad spectrum psychotropic agent, then to push it hard in primary care.
The ‘broad spectrum’ message works hand in glove with the insecurity of primary care physicians about making clear psychiatric diagnoses that require differentiated or specific treatments. Instead, the message is that one drug fits all and they can feel good about it! On this basis, some of the newer target disorders for Seroquel are OCD and generalized anxiety disorder.
The cynicism of this marketing approach becomes clear when we see how the duration-dependent side effects of a drug like Seroquel are downplayed while at the same time the issue of need for long term treatment is finessed. As one example, the 3-year incidence of tardive dyskinesia with Seroquel in patients with schizophrenia is 3.1%. That rate is substantially lower than with typical antipsychotic drugs (8.3% oral, 11.3% depot in the same report), but on the other hand when millions of patients are given a drug like Seroquel in primary care for depression or anxiety (that’s how billions in sales are generated) then we can expect to find 31,000 cases of TD for every million persons treated. First do no harm, especially considering how little good has been demonstrated.
The TD numbers come from Novick D et al. Journal of Clinical Psychopharmacology 2010;30:531-540.
Seroquel the wonder drug: AstraZeneca’s golden egg -all purpose! look at the drug trials! http://tinyurl.com/24vm5hk Menopausal depression, BLUSHING!, cocaine dependence! and more $$$$$ they are going to milk that patent for all its $$$
Yes, and thank youfor the link, Stephany. You were right out in front on this issue.