-
weight-neutral, placebo-like EPS, well-tolerated
-
superior antipsychotic efficacy
-
expanded indications• Clinical Trials with published studies• F.D.A. Approvals
-
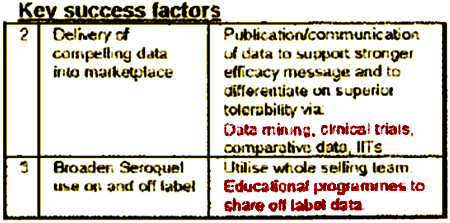
promoting off-label indications
For $520 Million, AstraZeneca Settles Case Over Marketing of a Drug
New York Times
By DUFF WILSON
April 27, 2010AstraZeneca has completed a deal to pay $520 million to settle federal investigations into marketing practices for its blockbuster drug, Seroquel, the Attorney General, , said at a news conference Tuesday afternoon. “AstraZeneca paid kickbacks to doctors as part of an illegal scheme to market drugs for unapproved uses,” , secretary of health and human services, said at the event in Washington. She said the company promoted drugs for unapproved uses by children, the elderly, veterans and prisoners.
Glenn Engelmann, AstraZeneca’s U.S. general counsel, released a statement saying the company denies the allegations but settled the investigation with the payment. “It is in the best interest of AstraZeneca to resolve these matters and to move forward with our business of discovering and developing important, life-changing medicines — while avoiding the delay, uncertainty, and expense of protracted litigation,” Mr. Engelmann said…
AstraZeneca agreed to sign a corporate integrity agreement with the federal government over its marketing of Seroquel for unapproved uses, but will not face criminal charges, company and federal officials said. The company, based in London, has been accused of misleading doctors and patients by playing up favorable research and not adequately disclosing studies that show Seroquel increases the risk of …
The company was facing two federal investigations and two whistle-blower lawsuits involving Seroquel sales and marketing practices. One of the investigations related to physicians who had participated in clinical trials. The other inquiry involved sales staff. As a result of aggressive marketing, Seroquel has been increasingly used for children and elderly people for indications not approved by the . The drugs have caused rapid weight gain in children, and side effects including deaths have prompted warnings against giving the drugs to elderly patients for dementia…
 And there’s a subtle version going on right now. In December 2009, Seroquel XR was approved for the adjunctive treatment of patients with Major Depressive Disorder who have not responded to antidepressants [Major Depressive Disorder]. No matter where you turn, you see some version of that ad on the right [this one was in the New York Times]. It doesn’t say "Adding SEROQUEL XR for Major Depressive Disorder may help." It says, "Adding SEROQUEL XR for depression may help." And that’s the way I see it being used in practice. People who say they are depressed get put on SSRIs with no attention to whether they meet any criteria for Major Depressive Disorder or anything else. When they don’t respond, they get SEROQUEL or SEROQUEL XR. They may be sad for a million perfectly understandable reasons – out of work, lost a friend, lonely, whatever. In essence, what that ad says is "Psychiatric complaint in busy doctor’s office equals SSRI for a bit, then SEROQUEL XR when it doesn’t work."
And there’s a subtle version going on right now. In December 2009, Seroquel XR was approved for the adjunctive treatment of patients with Major Depressive Disorder who have not responded to antidepressants [Major Depressive Disorder]. No matter where you turn, you see some version of that ad on the right [this one was in the New York Times]. It doesn’t say "Adding SEROQUEL XR for Major Depressive Disorder may help." It says, "Adding SEROQUEL XR for depression may help." And that’s the way I see it being used in practice. People who say they are depressed get put on SSRIs with no attention to whether they meet any criteria for Major Depressive Disorder or anything else. When they don’t respond, they get SEROQUEL or SEROQUEL XR. They may be sad for a million perfectly understandable reasons – out of work, lost a friend, lonely, whatever. In essence, what that ad says is "Psychiatric complaint in busy doctor’s office equals SSRI for a bit, then SEROQUEL XR when it doesn’t work."






What really upsets me is the “grease the skids for dementia” comment!
Why let data get in the way of a claim!
It’s also interesting how AZ’ s senior management were plugging Seroquel to Wall St: http://pharmagossip.blogspot.com/2011/02/what-were-astrazenecas-senior.html
WOW! Thanks for posting these insider documents; they give a fantastic picture of pharma’s machinations. This is really valuable stuff!
You mention that Astra-Zeneca is still at it; right you are! I wrote a little bit about that myself here. Atypical antipsychotics in general are being “renamed” as we speak; they’re soon to be “antidepressant augmentors.” So as public awareness of the dangers of antipsychotics rises, little to no awareness of the danger of augmentor drugs (the very same thing!) exists.
Ah, pharma… you are ever so clever.
Keep up the good blogging!