Most of the internal documents from AstraZeneca relate to the first half of the decade when the focus was on the competition among the Atypicals based on efficacy and adverse effects treating Schizophrenia – EPS, TD, weight gain, Diabetes, etc. By mid-decade [CATIE], clinical experience and the longer-term studies had pretty well clarified things for anyone who paid attention. Zyprexa was the most efficacious but caused the greatest weight gain and risk for Diabetes. Risperdal usually came in second in efficacy, but caused the most EPS and TD. Seroquel was the Atypical most likely to be discontinued, though in the middle in terms of side effects. None was the side effect free, potent antipsychotic of the ads [or our dreams]. And by 2004, the F.D.A. was requiring labeling warnings for the Metabolic Syndrome [Diabetes] and Tardive Dyskinesia, and was beginning to issue warning letters for off-label promotion.
AstraZeneca had planned to expand its indications into the Bipolar Disorders in the Seroquel Strategic Plan of 2000, and had been doing that in "off-label" promotions. But when the warning letter came and Zypreza was approved by the F.D.A. for Mania and Bipolar treatment, they initiated a number of clinical trials of Seroquel in Manic and Bipolar Disorder patients [referred to by some as the "me too" phenomenon]. Their approval soon followed. The "me too" followed in the Childhood Psychoses and with adjunct Major Depressive Disorder [for Seroquel XR].
| CLINICAL TRIALS | ||||||||||
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|
|
||||||||||
| Schizophrenia | 1 | 2 | 5 | 2 | 2 | 2 | 1 | 2 | ||
| Bipolar Disorders | 3 | 6 | 4 | 2 | 2 | 3 | 2 | |||
| Major Depression | 2 | 8 | 1 | 2 | 1 | |||||
| Anxiety Disorder | 1 | 5 | 1 | 1 | 2 | |||||
| Other | 1 | 1 | 4 | 4 | 2 | 3 | 3 | |||
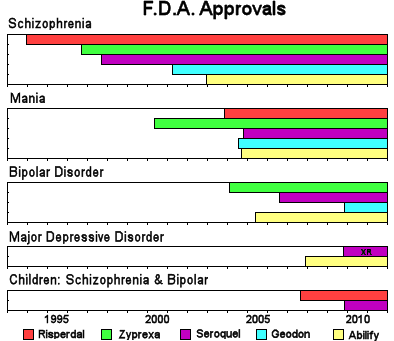
| While the wording of the F.D.A. Approvals varies from drug to drug, this chart captures the gist of the time-line of the approval for their various indications. |
People acquainted with how all this works immediately recognize the Clinical Trial formula used in getting these F.D.A. Approvals [described earlier – selling seroquel III: the data factories…].They all look the same: some rating scale plotted against time comparing Seroquel to placebo. The placebo group always gets better, and the drug adds a little something, sometimes more than others, but always significant. There are usually 50% drop-out rates, sometimes more. And the ITT/LOCF methodology makes the primary data opaque.
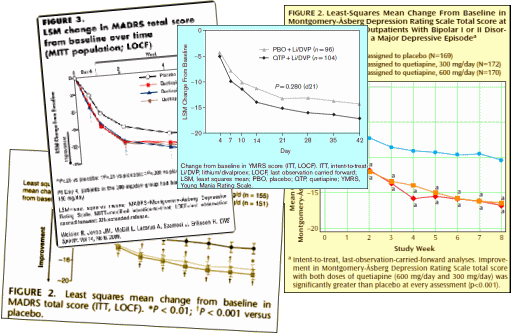
These clinical trials are funded by AstraZeneca, have a number of AstraZeneca employees as authors, are conducted by CRO’s selected and paid for by AstraZeneca, and are written by a medical writers hired by AstraZeneca. But the Psychiatrist authors were in the mix, recruited by AstraZeneca as well. Here’s an example where one of the "invited" authors balked:
Dr. Roger McIntyre suggested Dr. Nassir Ghaemi as a second author of a Clinical Trial paper comparing Haldol and Seroquel in treating Manic episodes. Obviously, the trial was over and the paper almost finished by the time he was asked. [To his credit] Dr. Ghaemi responded with:
This is an internal AstraZeneca email:
And one of the responses:
The paper was published in 2005 with Dr. McIntyre and four AstraZeneca employees as authors [no Dr. Ghaemi] [Quetiapine or haloperidol as monotherapy for bipolar mania – 12-week, double-blind, randomised, parallel-group, placebo-controlled trial]:
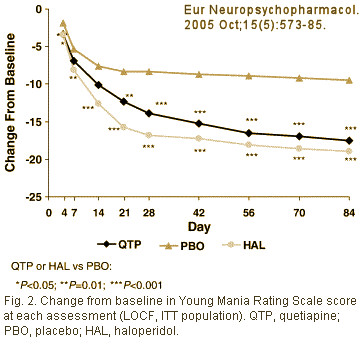
[Standard Clinical Trial fare ITT, LOCF, 50%+ drop-out] In case you’re wondering why the two people receiving the internal AstraZeneca email are in red, it’s because they’re the contract writers from Parexel who actually wrote the paper:
![]()



Sorry, the comment form is closed at this time.