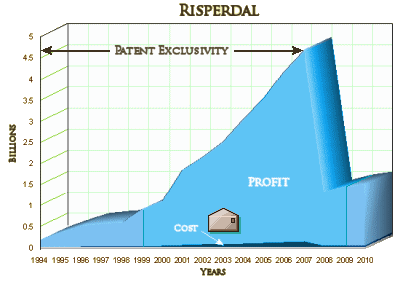
As with all the psychopharmacology blockbusters in the last twenty-five years, Risperdal [Janssen, Johnson & Johnson, Ortho-McNeil Janssen] was marketed deceptively. In the South Carolina penalty settlement the Judge noted that they had evidence that the manufacturer knew that Risperdal was associated with metabolic side-effects of some magnitude:

I can find no evidence of the studies mentioned above ["RIS USA-113 & 275 & ERI"] even now – they are presumably in the discovery material used in the trial but it’s not posted. Then, when instructed to send a "Dear Doctor" letter about those side effects in 2003, they sent out an advertisement instead:

They are appealing a $257 M settlement in Louisiana and are likely to appeal this $327 M settlement in South Carolina. Meanwhile, they are in negotiations with the Federal Government over a potential $1 B fine for off label-marketing. In November, they go to trial in Texas for an even more damning suit than any of these mentioned so far – manipulating the State by essentially bribing State and University officials to approve their drug for its Medicaid and other public medication programs, and then proselytizing the program to other States:
Sorry, the comment form is closed at this time.