 I periodically peruse the Coalition for Healthcare Communication web site. It’s essentially a proPHARMA organization that predictably attacks any attempt to reform any current practices. I visit it to see what they’re talking about. There’s a current commentary supporting a report by the Congressional Budget Office on Direct to Consumer advertising [those "ask your doctor if drug x is right for you…" ads]. In my new spirit of declaring personal biases, I hate those ads. Everyone around me hates those ads too, at least when I’m around, because I start talking to the television set. But I’m going to try to rise an inch or so above my automatic visceral reaction because I found the Congressional Budget Office report kind of interesting.
I periodically peruse the Coalition for Healthcare Communication web site. It’s essentially a proPHARMA organization that predictably attacks any attempt to reform any current practices. I visit it to see what they’re talking about. There’s a current commentary supporting a report by the Congressional Budget Office on Direct to Consumer advertising [those "ask your doctor if drug x is right for you…" ads]. In my new spirit of declaring personal biases, I hate those ads. Everyone around me hates those ads too, at least when I’m around, because I start talking to the television set. But I’m going to try to rise an inch or so above my automatic visceral reaction because I found the Congressional Budget Office report kind of interesting.
 The report studies a proposal by the Institute of Medicine to declare a moratorium on DTC advertising for the first two years after a new drug is approved. That actually seems like a sound idea – to give us a couple of years to see if undiscovered problems missed in the FDA approval process emerge. The CBO comes down opposing the idea [thus the CHC support]. What I found interesting were the stated reasons in the report. But first a Table from the report. Those ads cost a mint! – an impressive revenue stream for the media and quite an outlay for the manufacturer. The ads must be effective for the companies to pay that kind of ticket.
The report studies a proposal by the Institute of Medicine to declare a moratorium on DTC advertising for the first two years after a new drug is approved. That actually seems like a sound idea – to give us a couple of years to see if undiscovered problems missed in the FDA approval process emerge. The CBO comes down opposing the idea [thus the CHC support]. What I found interesting were the stated reasons in the report. But first a Table from the report. Those ads cost a mint! – an impressive revenue stream for the media and quite an outlay for the manufacturer. The ads must be effective for the companies to pay that kind of ticket.
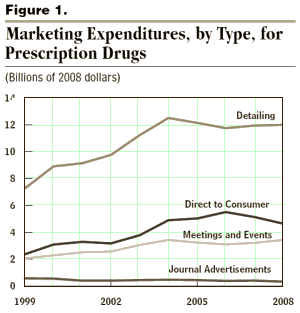
In 2008, spending on DTC advertising totaled $4.7 billion, nearly one-fourth of pharmaceutical manufacturers’ expenditures for all promotional activities. The rest of the $20.5 billion that pharmaceutical manufacturers spent on promotional activities in 2008 was directed at physicians and other health care professionals. In a practice called “detailing,” drug makers send representatives to visit physicians, nurse practitioners, and physicians’ assistants to discuss their products and to provide samples and reprints of academic literature on their company’s products. Pharmaceutical manufacturers also target physicians by advertising their drugs in medical journals and by sponsoring professional meetings and events, both in person and online.
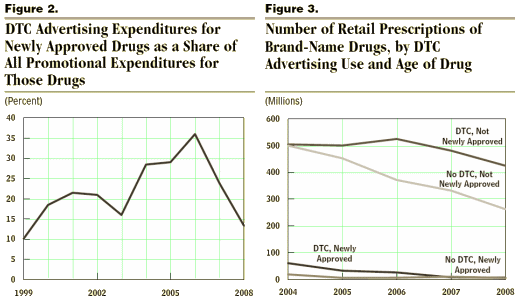
Over the 1999–2008 period, manufacturers varied their emphasis on consumers in their promotional spending for drugs during their first two years on the market (see Figure 2). That variation may have resulted in part from the relative newness of DTC advertising in the early part of that period because the FDA did not finalize its regulatory guidance on broadcast advertising for prescription drugs until August 1999.6 The changing characteristics of the new drugs approved by the FDA over the decade probably also contributed to the variation in the emphasis on DTC advertising over time. Between 2004 and 2006, when DTC advertising peaked as a share of promotional spending for new drugs, a large portion of the drugs in CBO’s data set that were less than two years old were counted among the top-selling drugs in the country. DTC advertising’s share of promotional spending for new drugs has declined since then because drugs approved more recently have not had such broad potential markets.
In 2007 and 2008, there were fewer newly approved drugs in CBO’s data set than in the preceding few years, and an even smaller number that were top sellers. Manufacturers submitted and the FDA approved fewer applications for new brand-name drugs than they had a decade earlier, and fewer still with large potential markets like a number of the drugs that obtained FDA approval in the 1990s.8 As a result, among the drug classes in CBO’s data set, new brand-name drugs made up a small and shrinking share of all retail sales of brand-name drugs, falling from nearly 10 percent in 1999 to less than 2 percent in 2008.The share of brand-name prescriptions attributed to drugs within their first two years after FDA approval exhibited a similar pattern, suggesting that lower sales, rather than declining drug prices, caused the drop in the share of retail sales for new drugs. New drugs accounted for 7 percent of brand-name prescriptions in 2004, but that share fell to less than 2 percent in 2008. The total number of brand-name prescriptions in CBO’s data set was dropping at the same time, from roughly 1 billion in 2004 to roughly 700 million in 2008, while the total number of prescriptions for generic drugs increased at a double-digit pace; during that period, patents expired for a number of older brand-name drugs, and prescriptions shifted to new generic versions of those drugs. The combination of a declining number of brand-name prescriptions and a declining share of those prescriptions representing new drugs meant that the number of prescriptions for new brand-name drugs plummeted. The number of prescriptions for newly approved brand-name drugs without DTC advertising fell from 19 million in 2004 to 5 million in 2008, and the number of prescriptions for newly approved brand-name drugs with DTC advertising dropped from 61 million in 2004 to 7 million in 2008 [see Figure 3].
Concerns about large numbers of patients taking drugs whose safety and possible side effects might not be fully understood underpin proposals to impose a two-year moratorium on DTC advertising for newly approved prescription drugs. Indeed, researchers have found a link between the promotional activities that pharmaceutical manufacturers use to expand the market for their drugs and increased reporting to the FDA of adverse events from a greater number of people taking those drugs. A moratorium on consumer advertising would provide more time for possible safety problems with some drugs to be uncovered and to become widely known.However, a moratorium on DTC advertising that delayed the widespread use of new drugs could also worsen — rather than enhance — public health in some ways. Positive effects on health from DTC advertising could be lost or delayed: Some studies have found that DTC advertising spurs individuals to seek treatment when they otherwise might not and improves patients’ compliance with prescribed drug regimens. Therefore, for drugs whose health benefits outweigh their safety and other concerns, a moratorium might reduce their use by a portion of the population who would benefit from the drugs. Moreover, in some cases, a moratorium on consumer advertising could postpone the realization of a drug’s true risks—if, for example, the number of people taking the drug was reduced enough by a moratorium that the full risks were not discovered as quickly.
I don’t like DTC advertising because the target audience has no way to really evaluate what they’re hearing. Some attractive older actress demonstrates her dancing skills after a fantasy recovery from fibromyalgia and the toxic possibilities are rapidly mumbled in the background. Another actress plays a depressed person and then laughs and plays with her pretend grandchildren because she’s added a long-acting antipsychotic while the downside drones on in a voice-over. That’s just not how I see medicine being practiced. But I don’t think we should use unproven speculations about public health to actively punish the pharmaceutical industry for sins past. There are enough non-speculative sins to put to an end. Our role should be to keep them honest, and to vigorously fight what they’ve done behind the scenes – not what they do in plain view.
And here I was thinking I was crazy for reading peer reviewed journals for fun. Seems I’m not the only one.
Don’t be a coward! I KNOW that you know that you are intelligent enough to answer my question. You just won’t do it publicly.
Nooooooooo!! Don’t shut the blog down just because some yahoo misses the point. Your work here is top notch, I read every word.
I, for one, would be very disappointed to lose your voice in these matters.
It’s quite understandable that you would want nothing to do with the hasslers, who do plague such open forums. You might consider blogging without an open comments section. WordPress allows you to turn that off. Alternatively, you might want to moderate all comments before they’re posted, though that might not be sufficient solution.
One of the crucial part of the craft of blogging is finding some way to deal with commentary (which can be powerfully aversive even when well meant!) that allows the blogger to continue blogging. It is a matter of setting boundaries so the writer can continue to function as a writer. And simply turning off comments is a perfectly legitimate way to do that. If you wish to have reader input, provide an email address or contact form for people to write you directly; there’s no reason for you to provide the rest of us with a soap box for our own agendas. We can all go get our own (perfectly free) blogs if we want to spout off.
In any event, whatever you chose, I wish you the best in your endeavors. Please feel free to use my email address to contact me, if so moved; I would certainly be happy to speak with you about your options as a blogger, both social and technical. Your words are welcome in my house.
@Patient 007,
I think the doctor here is intelligent enough to realise that he is a free man and thus, if he doesn’t want to answer your questions (and instead, spend his time on more creative endeavours…), he is free to do so. mind asking yourself the question; what would he gain by answering your questions?
@doctor,
I second Minder and may I suggest having password protected comment form? Of course, it is a drag to approve many subscribers but that could be a solution.
A.L.
Mickey, I beg of you, don’t be discouraged by some dam fools porting in from the Internet. Your work is brilliant and we so need your voice!
Mickey,
I totally 2nd Alto’s comments.
I 3rd Alto’s comments! Your blog has just “lost its innocence,” so to speak, and has attracted a troll. It was only a matter of time until this happened. It happens to all blogs and message boards eventually. Par for the course.
Really, your work is just too important to stop because of some keyboard commando looking to get a rise out of you.
Please continue on, even if you disable comments.
I really don’t understand what the issue is here. ALL of the top flight, AAA-rated (by Standard and Poors, no less!) psychiatry, medical, health care, and neuroscience cites allow for the blogger to censure comments that are offensive or even just off topic. Why don’t you just do the same? At the risk of sounding like Joe Biden vis a vis the Tea Party, are you going to let a “terrorist” take you down? On come on! Your work is too important. And its high time you realized that.
Please don’t quit. Your reasoning is sound and your attempts at filtering the facts out of all the Pharma stuff for regular people is invaluable.
If you need some help sorting out how to set things up to better manage comments, I am happy to help. I’m a sysadmin by trade, so there’s no shortage of help I can offer.
You have my email, it and the offer to help are real.
…then the trolls win. sorry to see this blog go down; one of the best psychiatry blogs on the internet.