Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses.
by Gueorguieva R, Mallinckrodt C, and Krystal JH.
Archives of General Psychiatry. 2011 68(12):1227-37.
CONTEXT: The high percentage of failed clinical trials in depression may be due to high placebo response rates and the failure of standard statistical approaches to capture heterogeneity in treatment response.
OBJECTIVE: To assess whether growth mixture modeling can provide insights into antidepressant and placebo responses in clinical trials of patients with major depression.
DESIGN: We reanalyzed clinical trials of duloxetine to identify distinct trajectories of Hamilton Scale for Depression (HAM-D) scores during treatment. We analyzed the trajectories in the entire sample and then separately in all active arms and in all placebo arms. Effects of duloxetine hydrochloride, selective serotonin reuptake inhibitor (SSRI), and covariates on the probability of following a particular trajectory were assessed. Outcomes in different trajectories were compared using mixed-effects models.
SETTING: Seven randomized double-blind clinical trials of duloxetine vs placebo and comparator SSRI. Patients A total of 2515 patients with major depression.
INTERVENTIONS: Duloxetine and comparator SSRI. Main Outcome Measure Total score on the HAM-D.
RESULTS: In the entire sample and in the antidepressant-treated subsample, we identified trajectories of responders (76.3% of the sample) and nonresponders (23.7% of the sample). However, placebo-treated patients were characterized by a single response trajectory. Duloxetine and SSRI did not differ in efficacy, and compared with placebo they significantly decreased the odds of following the nonresponder trajectory. Antidepressant responders had significantly better HAM-D scores over time than placebo-treated patients, but antidepressant nonresponders had significantly worse HAM-D scores over time than the placebo-treated patients.
CONCLUSIONS: Most patients treated with serotonergic antidepressants showed a clinical trajectory over time that is superior to that of placebo-treated patients. However, some patients receiving these medications did more poorly than patients receiving placebo. These data highlight the importance of ongoing monitoring of medication risks and benefits during serotonergic antidepressant treatment. They should further stimulate the search for biomarkers or other predictors of responder status in guiding antidepressant treatment.
TRIAL REGISTRATION: clinicaltrials.gov Identifier: NCT00073411.
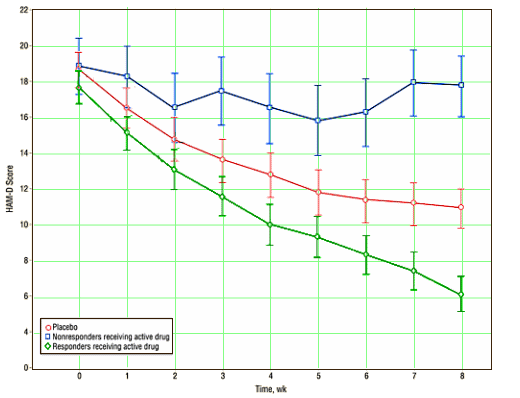
[graphs colored from the original for clarity]
This study is a reanalysis of seven placebo controlled clinical trials of Cymbalta [Duloxetine] with active comparators [Prozac, Paxil, and Lexapro]. They analyzed the data looking for trajectories – meaning they used mathematical methods to look for separate populations within the data. When they lumped all the data from the study drug, the active comparators, and the placebo together, they found that the data [HAM-D] fit a two population model. When they looked at the placebo data alone, it fit a one population model. There was no significant difference between the study drug and the active comparators, so they lumped all the active data together and again found that a two population model.
The graph above shows the Placebo data [red] and the drug data divided into the two populations [blue and green]. The study was not funded by Eli Lilly, the manufacturer of Duloxetine, but one author is a Lilly research scientist [Mallinckrodt] and one lists being on Lilly’s Advisory Board in his financial disclosure [Krystal]. The first author [Gueorguieva], a Yale Biostatistician, lists no conflicts of interest. In spite of the industry ties, I thought the analysis was both clever and interesting. There’s a big question about why the placebo response in Clinical Trials is so pronounced. There’s an equally big question about why it’s so difficult the get a significant study for drug effect. Most of us assume that the answer is in the method of selecting subjects. And while this study doesn’t change that, it does show something of interest – that the drug treated subjects seem to have two response patterns for responders and non-responders – neither looking like the placebo treated group – the majority [73%] looking better and a minority looking worse [23%] in a specific way. Obviously, the net effect is to move the average drug response towards the placebo group.
In so far as I’m capable of evaluating such a thing, I thought the analysis looked rational though it relies on some relatively heady mathematics. It struck me as familiar, reminding me of another study that I reviewed earlier [by people with no drug industry ties] reanalyzing the STAR*D raw data [Growth Modeling With Nonignorable Dropout: Alternative Analyses of the STAR*D Antidepressant Trial] [what’s missing…]. Their analysis was somewhat different in part because STAR*D had no placebo group, but their population analysis showed a similar group they called "the U-shaped class" because of the shape of their response curves. They were proposing using this kind of population analysis rather than the conventional LOCF and Mixed Model correction methods to deal with drop-outs, something mentioned in the current article as well.
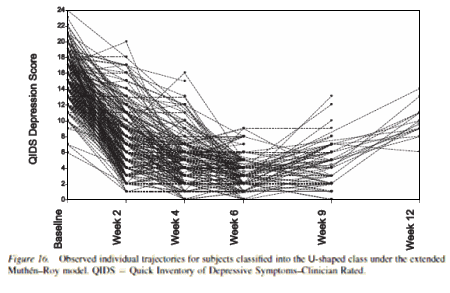
These results are due to the fact that Major Depressive Disorder is multiply-realized. It is a final common endpoint for various pathological mechanisms. Some biological ones that involve signaling cascades triggered by serotonin and/or norepinephrine binding may be more responsive to SSRI/SNRI medications. Others, involving other biological pathways, won’t, and may respond better to dopamine agonists, thyroid hormone, etc. Some MDD is the surface/superficial result of characterological dysfunction a la psychodynamics. Etc, etc, etc. The point is that the MDD diagnosis (and many other DSM diagnoses are superficial, quick-and-dirty-enough-that-I-can-grok-it-in-a-15-minute-med-check) is an expedient only, often not the full story (and I am not saying anything new, which none of you all don’t already know). The “non-responders” likely have just another “kind” of MDD that needs another “kind” of treatment.
Usual,
You can say that anytime you think it. I wish more people would. It just happens to be true…
Three studies worth reading –
http://www.ahrp.org/cms/content/view/831/96/
Duane
Cue the music for today’s study meta analysis. Variation on a tweet theme:
When you wish upon a STAR(*D),
Makes no difference who you are,
Anything your heart desires,
Won’t make a difference in MDD.
I don’t know how you can assume the study population had MDD; it is a “heterogeneous state” that because of the vagaries of diagnosis, often includes people without MDD. Measurements of treatment “response” are likewise flawed.
Neuroskeptic questions the data analysis methodology here http://neuroskeptic.blogspot.com/2011/12/do-antidepressants-make-some-people.html
Lilly was most certainly involved: “Author Affiliations: Division of Biostatistics, School of Public Health (Dr Gueorguieva), and Department of Psychiatry (Dr Krystal), Yale University School of Medicine, New Haven, and Veterans Affairs National Center for Posttraumatic Stress Disorder, Veterans Affairs Connecticut Healthcare System, West Haven (Dr Krystal), Connecticut; and Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, Indiana (Dr Mallinckrodt).”
“….Dr Krystal is on the scientific advisory board of Abbott Laboratories, Bristol-Myers Squibb, Eisasi Inc, Eli Lilly and Co, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharaceuticals Inc, Naurex Inc, Pfizer Pharmaceuticals, and Shire Pharmaceuticals….”
The paper concludes with a plea for more research into “personalized” medicine. That it threw Cymbalta under the bus — well, it’s EOL in 2003. The future belongs to the atypicals, anyway. (The campaign undermining antidepressants in order to better position atypicals for depression drives David Allen crazy http://davidmallenmd.blogspot.com/2011/08/plausible-deniability.html )
And, may I add, withdrawal syndrome as a confounding factor raises its head yet again: “The inferences drawn from the present analyses are limited by several factors. First, we were unable to differentiate whether the poor responses to antidepressants arose from a negative effect of taking antidepressant medication or as a consequence of discontinuation of these medications.“