J&J to Agree to $1B Accord in Risperdal Probe
Bloomberg
By Margaret Cronin Fisk, Jef Feeley and David Voreacos
January 5, 2012
Johnson & Johnson (JNJ) will pay more than $1 billion to the U.S. and most states to resolve a civil investigation into marketing of the antipsychotic Risperdal, according to people familiar with the matter. J&J, the world’s largest health products company, reached an accord last week with the U.S. attorney in Philadelphia, according to the people, who weren’t authorized to speak about the matter. It doesn’t resolve negotiations over a possible criminal plea, they said. The U.S. government has been investigating Risperdal sales practices since 2004, including allegations the company marketed the drug for unapproved uses, J&J has said in Securities and Exchange Commission filings (JNJ). The company said it has been in negotiations with the U.S. to settle this investigation. J&J, based in New Brunswick, New Jersey, disclosed in August that it reached an agreement to settle a misdemeanor criminal charge related to Risperdal marketing. The company is in negotiations to pay about $400 million more to settle this portion of the investigation, one of the people said. “We’re not going to comment on rumor or speculation,” Teresa Mueller, a J&J spokeswoman, said in a phone interview. Company officials said in an SEC filing in May that they had reserved funds to resolve the government’s claims over Risperdal marketing. The company didn’t say how much had been set aside. The drugmaker said in an August filing it added an unspecified amount to the reserve to cover criminal penalties.Accord Announcement: When the final settlement will be announced isn’t clear. The Justice Department typically announces civil and criminal resolutions at the same time in corporate cases. A majority of U.S. states will join the settlement, the people said. Which ones will accept the final agreement hasn’t been determined, they said. Each state can decide whether to join the federal government’s settlement or pursue its own case. Typically, states with cases in court continue to pursue their own. Texas alone is asking for more than $1 billion in a case that goes to trial next week. Risperdal, which was first approved by the Food and Drug Administration in 1993, later became J&J’s best-selling drug. Worldwide sales totaled $24.2 billion from 2003 to 2010, reaching $4.5 billion in 2007. After that, J&J lost patent protection and sales declined. J&J and its Janssen unit, which sold Risperdal, deny any wrongdoing in the Texas case.
‘Vigorously Defend Itself’: “Janssen is prepared to vigorously defend itself against these claims,” Mueller said in an e-mail. “We are committed to ethical business practices, and have policies in place to ensure that our products are only promoted for their FDA-approved indication.” J&J and its Janssen unit have been sued by 12 states, including Texas, South Carolina and Louisiana, over Risperdal marketing. The attorneys general of the other states “have indicated a potential interest in pursuing similar litigation against” Janssen, J&J said in its quarterly SEC filing in November. A jury in Louisiana, weighing claims that the company downplayed the drug’s risks, awarded that state $257.7 million in 2010. A South Carolina judge last year ordered J&J to pay $327 million over Risperdal sold in the state. The FDA approved Risperdal in 1993 for psychotic disorders including schizophrenia. That market is limited, and Janssen sought to sell Risperdal for bipolar disorder, dementia, mood and anxiety disorders and other unapproved uses, according to documents in the lawsuit by the state of Louisiana.
Unapproved Uses: Hundreds of Janssen salespeople sold to doctors, nursing homes, Veteran’s Administration facilities and jails, the records show. Marketers gave doctors materials about studies of unapproved uses for Risperdal. Janssen sponsored clinical trials of the drug’s effect on other illnesses. In 1994, 1999 and 2004, the FDA ordered Janssen to stop making false and misleading marketing claims about Risperdal’s superiority. The FDA told J&J in 1999 that its marketing materials for geriatric patients overstated Risperdal’s benefits and minimized risks. A J&J business plan for the next year called for increasing the drug’s market share for elderly dementia sales, an unapproved use, according to documents in the Louisiana lawsuit. The FDA didn’t approve Risperdal for bipolar disorder until 2003. In 2006, the regulator approved it for symptoms related to autism in children and teens. The FDA approved it to treat bipolar children and teens the next year. It was never approved for dementia…
There are a finite number of people with Schizophrenia, and many Schizophrenic patients are treated in the public mental health system paid for by government funds – State and Federal. The pages of this blog are filled with the sordid story of how J&J used stealth marketing to get their drug used in these systems, hopefully something that will be even clearer in the Texas Trial next week where State officials were used to bilko the States they worked for. In addition, J&J came out of the gate marketing Risperdal inappropriately for a much wider swath of mental health patients – including small children, adults with a wide variety of diagnoses, and even the elderly. In my opinion, their methods were criminal, compromising the integrity of the academic medicine and the medical system at its core. I don’t think I’m overstating this at all. The FDA saw what was happening and ordered J&J to stop its corrupt marketing – and for all intents and purposes, J&J ignored the orders repeatedly. Here’s my often shown graph of why:
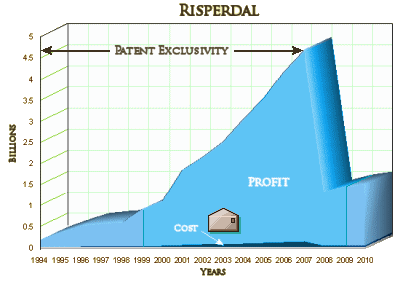
The issue is not that they made a bundle. It’s that they made a bundle through active fraud – fraudulent claims of efficacy, fraudulent claims of superiority, fraudulent claims for safety, fraudulent claims for indication, fraudulent omission of toxicity [and, by the way, plenty of people were hurt by taking the drug]. They bought off some physicians along the way, but they misinformed a much larger number by flooding medical education and medical literature with a whole lot of pseudo-experts and pseudo-science.
what percentage of $1 billion is that for the total profits J&J has made on Risperdal and any related products like Invega these past almost 20 years since it first came out? My guess, at least $20 billion, as 5% is a drop in the bucket for that profit margin. When will people who have true judicial and financial impact on companies like Big Pharma going to realize fining them pittance amounts do not send a message other than this: as long as there are no real painful and long lasting consequences for screwing the public, don’t ever change your ways!
I’d also love to know what Dr. Hassman asked “what pecentage of $1 billion is that for the total profits J&J has made on Risperdal****
Today is Jan 19,’12 and the settlement in TX was for just $158M.