It hasn’t been the best of times, and we’re not far enough beyond to yet know if it has been the worst of times. All we really know is that it has been our times. I suppose that it’s always that way – one’s personal slice of history hasn’t really yet become history proper, it’s still officially the present. So editorial perspective remains heavily colored by the personal experience of the people who have lived it.
As a physician psychiatrist, I’ve seen a lot of depressed people over the years, and the recommendations about how to help them have been a potpourri, at the least. Like most of us, a lot of what I’ve done has increasingly relied on the intuition of experience – successes, failures, mistakes – anecdotes they’re called [sometimes warmly and sometimes as an invective].
So, what is a clinician to do? If we pay attention to the epidemiologic data, melancholia in its unipolar and bipolar forms affects maybe 3–4% of the population at most, whereas estimates of MDD prevalence range up to 25% or higher. The default position has to be, do not miss cases of melancholia; learn how to recognize them sooner rather than later in their course; do not fail to treat them with a genuinely effective agent; and think hard before casually prescribing antidepressant drugs to nonmelancholic patients with MDD because that may be a formula for wasted resources, unnecessary side effects, and unproductive clinical management.
Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database.
by Khan A, Leventhal RM, Khan SR, and Brown WA.
Journal of Clinical Psychopharmacology 2002 22[1]:40-5.
Some studies suggest that more severely ill patients with depression respond well to antidepressants and poorly to placebo, whereas those who are mildly ill respond equally well to antidepressants and placebo. This notion has implications for the design of clinical trials. To further assess and substantiate these putative predictors of antidepressant and placebo response, we assessed the Food and Drug Administration database of 45 phase II and III antidepressant clinical trials. The frequency of statistically significant differences between antidepressants and placebo was higher in the trials that included patients with more severe depression. In the antidepressant-treated groups, the magnitude of symptom reduction was significantly related to mean initial Hamilton Rating Scale for Depression [HAM-D] score; the higher the mean initial HAM-D score, the larger the change. With placebo treatment, however, the higher the mean initial HAM-D score, the smaller the change. Early discontinuation was more frequent among patients whose mean initial HAM-D scores were higher. These data may help inform the design of future antidepressant clinical trials.
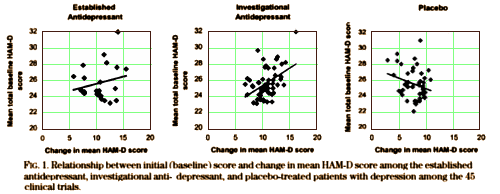
Our results suggest that the severity of depressive symptoms, as measured by mean initial HAM-D score, influences the outcome of randomized, double blind, placebo-controlled antidepressant clinical trials. Antidepressants’ effects seem more robust and placebos’ less so among patients with more severe depression.
Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration
by Irving Kirsch, Brett J. Deacon, Tania B. Huedo-Medina, Alan Scoboria, Thomas J. Moore, and Blair T. Johnson
PLoS Medicine. 2008 5[2]: e45.
[full text on-line]
Background: Meta-analyses of antidepressant medications have reported only modest benefits over placebo treatment, and when unpublished trial data are included, the benefit falls below accepted criteria for clinical significance. Yet, the efficacy of the antidepressants may also depend on the severity of initial depression scores. The purpose of this analysis is to establish the relation of baseline severity and antidepressant efficacy using a relevant dataset of published and unpublished clinical trials.
Methods and Findings:We obtained data on all clinical trials submitted to the US Food and Drug Administration (FDA) for the licensing of the four new-generation antidepressants for which full datasets were available. We then used meta-analytic techniques to assess linear and quadratic effects of initial severity on improvement scores for drug and placebo groups and on drug–placebo difference scores. Drug–placebo differences increased as a function of initial severity, rising from virtually no difference at moderate levels of initial depression to a relatively small difference for patients with very severe depression, reaching conventional criteria for clinical significance only for patients at the upper end of the very severely depressed category. Meta-regression analyses indicated that the relation of baseline severity and improvement was curvilinear in drug groups and showed a strong, negative linear component in placebo groups.
Conclusions: Drug–placebo differences in antidepressant efficacy increase as a function of baseline severity, but are relatively small even for severely depressed patients. The relationship between initial severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among very severely depressed patients, rather than to increased responsiveness to medication.
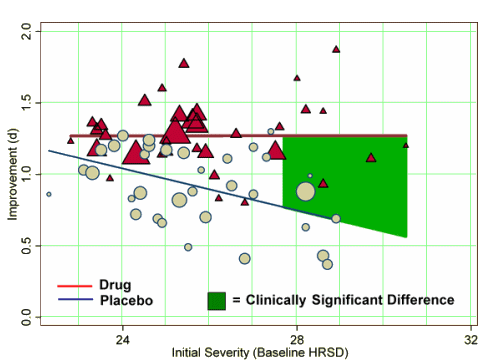
… although differences in improvement increased at higher levels of initial depression, there was a negative relation between severity and the placebo response, whereas there was no difference between those with relatively low and relatively high initial depression in their response to drug. Thus, the increased benefit for extremely depressed patients seems attributable to a decrease in responsiveness to placebo, rather than an increase in responsiveness to medication.
 So, back to the future. I expect that the SSRI/SNRI drugs will stay with us into the Age of the Generics – the short half-life drugs fading away because of their difficult withdrawal potential. They’ll be thought of as symptomatic drugs, useful in cases of severe depression, and we’ll be careful when we use them to watch for akathisia and suicidality early on. In the best of worlds, they’ll be used in the context of a comprehensive approach to mental health care rather than as the only treatment modality available. And hopefully, we’ll all be on the lookout for the less common melancholics and help them get the kind of treatment they deserve as Dr. Carroll suggests. Not so very different from how things were in 1979…
So, back to the future. I expect that the SSRI/SNRI drugs will stay with us into the Age of the Generics – the short half-life drugs fading away because of their difficult withdrawal potential. They’ll be thought of as symptomatic drugs, useful in cases of severe depression, and we’ll be careful when we use them to watch for akathisia and suicidality early on. In the best of worlds, they’ll be used in the context of a comprehensive approach to mental health care rather than as the only treatment modality available. And hopefully, we’ll all be on the lookout for the less common melancholics and help them get the kind of treatment they deserve as Dr. Carroll suggests. Not so very different from how things were in 1979…
I attended Drummond Rennie’s talk last evening at the Harvard Law School. His “old boss”, former NEJM editor, Bud Relman, was in the front row. Lawrence Lessig offered the introduction. Then Dr. Rennie was off to the races. I wish you and the likes of Dr. Carroll and other readers and commenters had been there, as well.
He was blunt. He was honest. His message was starkly dark and dangerous.
It was brilliant.
Mickey,
I think you might be interested in this Stewart et. al. article (see below) and I’d appreciate your comments on it. In the press it has been described as showing “Medication helps some with mild depression”. Like this:
Medication helps some with mild depression
Medication could help mild depression, say researchers
…and finally…
Study: People Who Suffer Mild Depression Should Take Meds
I’d imagine that most people would take this to mean that SSRIs/SNRIs are effective in mild depression (although the press accounts don’t actually say that):
HDRS:
0-7 = Normal
8-13 = Mild Depression
14-18 = Moderate Depression
19-22 = Severe Depression
>= 23 = Very Severe Depression
But the actual study seems to be mostly about something other than “mild depression” and SSRIs, even if the authors themselves refer to their pre-existing conviction that antidepressants are effective in “mild depression” in their abstract.
Stewart, JA et. al. (2011) Can People With Nonsevere Major Depression Benefit
from Antidepressant Medication?, J. Clin. Pyshciatr. (on line)
This is a review of six double-blind placebo controlled studies of antidepressants, but excluding patients with HDRS ratings >23. Looking at the full article it appears to me that around 2/3 of the patients actually were treated with tricyclics, the patient mix included patients with severe, moderate and mild depression and the only one of the six studies which included an SSRI excluded some patients with “mild” depression. How do you think the press accounts square with the published paper, and if this is spin, who would you guess is responsible?