Major depressive disorder: new clinical, neurobiological, and treatment perspectives
Seminar in The Lancet
by David J Kupfer, Ellen Frank, Mary L Phillips
December 20, 2011
In this Seminar we discuss developments from the past 5 years in the diagnosis, neurobiology, and treatment of major depressive disorder. For diagnosis, psychiatric and medical comorbidity have been emphasised as important factors in improving the appropriate assessment and management of depression. Advances in neurobiology have also increased, and we aim to indicate genetic, molecular, and neuroimaging studies that are relevant for assessment and treatment selection of this disorder. Further studies of depression-specific psychotherapies, the continued application of antidepressants, the development of new treatment compounds, and the status of new somatic treatments are also discussed. We address two treatment-related issues: suicide risk with selective serotonin reuptake inhibitors, and the safety of antidepressants in pregnancy. Although clear advances have been made, no fully satisfactory treatments for major depression are available.
Worldwide, depression is a seriously disabling public health problem of very high prevalence. Major depressive disorder has a 12-month prevalence of 6·6% and a lifetime prevalence of 16·2%, is twice as common in women as in men, and causes considerable impairment. Age-of-onset distributions suggest that depression is prevalent for the entire lifespan. The disorder not only produces decrements in health that are equivalent to those of other chronic diseases [eg, angina, arthritis, asthma, and diabetes], but also worsens mean health scores substantially more when comorbid with these diseases, than when the diseases occur alone. A crucial implication is that primary care providers should not ignore the presence of depression when patients have a chronic physical disorder.
Another advance is the introduction of agomelatine—a melatonin [MT1 and MT2] agonist and a 5-HT2C-receptor antagonist. Agomelatine has shown a generally favourable tolerability and efficacy, therefore providing a promising alternative for patients who do not respond to existing pharmacotherapies, or who cannot tolerate their side effects. Placebo-controlled research provides evidence for the effectiveness of agomelatine as both an acute and a continuation treatment for major depression.
But enough of Hickie, this seminar of Kupfer’s has much more to offer. So he starts with a pitch for "genetic, molecular, and neuroimaging studies that are relevant for assessment and treatment selection of this disorder." He cuts and pastes the world-health-organization-dire-prediction-about-the-burden-of-depression piece in the beginning, then elaborates the advances in "genetic, molecular, and neuroimaging" part for a few pages as expected [it does say neurobiology in the title]. But when he gets to treatment, Agomelatine isn’t his only foible. He reports on the STAR*D Study:
Since the latest update on depression in The Lancet, the results of the sequenced treatment alternatives to relieve depression [STAR*D] study—the largest depression study ever done outside the pharmaceutical industry—have been reported. This study was a practical clinical trial with broad inclusion criteria, resulting in a highly representative sample of the US population. Undertaken in both psychiatric and primary care settings, STAR*D used up to four successive treatment steps, including a switch to and augmentation with additional drug or cognitive therapy in an equipoise randomisation design. The goal was for full remission, rather than just response. Remission rates in steps one to four were disappointing at 36.8%, 30.6%, 13.7%, and 13.0%, respectively, with a cumulative remission rate of 67% after all four steps. These rates were low compared with efficacy trials of antidepressants, which suggests that, in actual practice, most patients need several sequential treatment steps to achieve remission. The STAR*D trial showed no clear advantage of one strategy of drug over another for patients who did not achieve remission after one or more acute treatments. Furthermore, because there was no placebo control in this hybrid [efficacy effectiveness] trial, there is no way to know whether any of the strategies were better than maintenance of the original treatment for an additional period. Too few patients received psychotherapy, either as an augmentation or a switch strategy, to make firm conclusions about its role. Neither sociodemographic nor clinical [anxious, atypical, and melancholic] features moderated the effect of various switching options after the first non-successful attempt at acute treatment. No differences in outcomes were found between primary care and psychiatric settings in the first two stages of acute treatment, suggesting that primary care physicians can be reasonable providers of care for patients with less complex depression.
-
Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. by Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, and Kupfer DJ. in the Psychiatric Clinics of North America. 2003 26(2):457-94.
-
Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. by Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G; STAR*D Investigators Group. in Control Clinical Trials. 2004 25(1):119-42.
-
Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. by Hollon SD, Shelton RC, Wisniewski S, Warden D, Biggs MM, Friedman ES, Husain M, Kupfer DJ, Nierenberg AA, Petersen TJ, Shores-Wilson K, and Rush AJ. in the Journal of Psychiatric Research. 2006 40(1):59-69.
-
Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. by Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, and Fava M. in the American Journal of Psychiatry. 2006 163(11):1905-17.
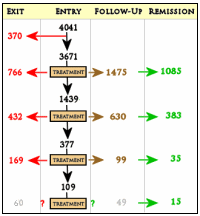 This is hardly a rich full summary of STAR*D as it actually happened. He leaves out that the essential motif of the study was how to chase people off, considering their dramatic drop out rates [right]. He omits the fact that a central goal of the study was cost analysis of treatment which has never been reported [or even mentioned] in the 100+ papers from this study. For that matter, they never published the primary and secondary outcomes either – HAM-D or IDS-C – relying instead on the QIDS-SR [a self report rating scale that was itself experimental] because that’s the only data their faulty design made it possible for them to collect [see a thirty-five million dollar misunderstanding…]. About those remission rates, they were fantasy remission rates – what might have been had no one dropped out [but 40% did]. The highest possible estimate of their remission rate would be 40% [with no placebo group to compare rates with], and it was actually probably much lower. Yet undaunted by failure, the STAR*D papers continue to be published.
This is hardly a rich full summary of STAR*D as it actually happened. He leaves out that the essential motif of the study was how to chase people off, considering their dramatic drop out rates [right]. He omits the fact that a central goal of the study was cost analysis of treatment which has never been reported [or even mentioned] in the 100+ papers from this study. For that matter, they never published the primary and secondary outcomes either – HAM-D or IDS-C – relying instead on the QIDS-SR [a self report rating scale that was itself experimental] because that’s the only data their faulty design made it possible for them to collect [see a thirty-five million dollar misunderstanding…]. About those remission rates, they were fantasy remission rates – what might have been had no one dropped out [but 40% did]. The highest possible estimate of their remission rate would be 40% [with no placebo group to compare rates with], and it was actually probably much lower. Yet undaunted by failure, the STAR*D papers continue to be published.
And as for the similarities between primary care and psychiatric sessions, the subjects were treated in both by the clinical research coordinator who did a QIDS-C and other scales, then the preassigned medication was adjusted by a clinician, and then they did the QIDS-SR all by themselves [hardly a "face off"]. Little wonder there was no difference [a cleaned up parking-lot attendant or Trivedi’s computer could’ve filled in].
Increased data for imaging and genetics in major depressive disorder, and other neurobiological data, provide potential biomarkers for the assessment of treatment outcomes. If a description of precise subgroups based on such data were to emerge, short-term and long-term benefits of treatment might be improved. Although new reports about treatment response in multisite studies have emerged in the past 5 years, treatment advances are somewhat lagging because of an inability to undertake adequate studies with the appropriate predictors of response.
-
Although clear advances have been made, no fully satisfactory treatments for major depression are available.
There’s another way to look at this. There is no such thing as Major Depressive Disorder. It is, by any parameters, a heterogeneous collection of conditions – known and unknown. It would be more accurate to say "no fully satisfactory evidences for major depression are available." As facetious as that sounds, I mean it in earnest and facetiously -
Increased data for imaging and genetics in major depressive disorder, and other neurobiological data, provide potential biomarkers for the assessment of treatment outcomes.
While that sentence is over-stated, missing a "maybe" or "possibly" here and there, we all hope that biomarkers for some distinct syndromes within the collage of MDD will come along some day. Who wouldn’t want that? Call us when and if it happens. -
If a description of precise subgroups based on such data were to emerge, short-term and long-term benefits of treatment might be improved.
How about the "precise subgroups" we already have. In the biological realm, melancholia in it’s various flavors in a good place to start. Since there’s no evidence that it is on a spectrum with other depressions, how about separating it out so the researchers will have a distinct entity to work with? How about the cases where the depression is clearly linked to personality disorder? How about post-partum depression? Why wait on fantacized neurobiology? Why not seize the Moment? -
Although new reports about treatment response in multisite studies have emerged in the past 5 years, treatment advances are somewhat lagging because of an inability to undertake adequate studies with the appropriate predictors of response.
Ditto the above. If he’s talking about STAR*D as the "treatment response in multisite studies" "in the past 5 years," the problems were actually that it was not an "adequate stud[y]." It was poorly administered, poorly designed, largely unintelligible beyond level 1, if even that. But Kupfer’s lament about the absence of "appropriate predictors of response" deserves further comment. He’s assuming that there will be such things – some biologically measurable things – a gene, a protein, a hormone, a neuro-image that says Prozac, or Pristiq, or Wellbutrin. So far, there’s no compelling evidence that there is even a differential among medications. Is there even a biological difference between responders and non-responders? More likely, but yet unproven and not likely to be proven with the current heterogeneity in the diagnostic criteria.
Sorry, the comment form is closed at this time.