Mudhukar Trivedi, M.D., director of the mood disorders research program at the University of Texas Southwestern, observed that “we are creating a virtual world in which [psychiatric] trials are conducted in the [least-severely ill] patients by the least-qualified people, and then [we] are surprised by the placebo effect.” Consequently, the general public and part of the medical community believe that antidepressants are no better than placebo.
Randomized, Placebo-Controlled Trials of Antidepressants for Acute Major Depression: Thirty-Year Meta-Analytic Review
by Juan Undurraga and Ross J Baldessarini
Neuropsychopharmacology [advance online publication]
14 December 2011
Abstract: Antidepressant–placebo response-differences [RDs] in controlled trials have been declining, potentially confounding comparisons among older and newer drugs. For clinically employed antidepressants, we carried out a meta-analytic review of placebo-controlled trials in acute, unipolar, major depressive episodes reported over the past three decades to compare efficacy [drug–placebo RDs] of individual antidepressants and classes, and to consider factors associated with year-of-reporting by bivariate and multivariate regression modeling. Observed drug–placebo differences were moderate and generally similar among specific drugs, but larger among older antidepressants, notably tricyclics, than most newer agents. This outcome parallels selective increases in placebo-associated responses as trial-size has increased in recent years. Study findings generally support moderate efficacy of clinically employed antidepressants for acute major depression, but underscore limitations of meta-analyses of controlled trials for ranking drugs by efficacy. We suggest that efficiency and drug–placebo differences may be improved with fewer sites and subjects, and better quality-control of diagnostic and clinical assessments.
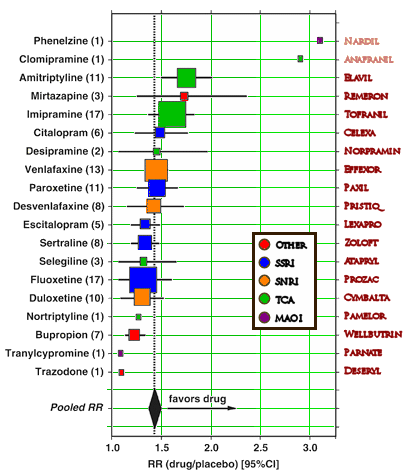
Undurraga and Baldessarini did a meta-analysis of the antidepressant drug trials for the last thirty years, ending up with only 116 candidates from over 2000 studies that met their criteria [placebo controlled, randomized, double blind trials]. They used the study RR [Response Rate] for comparison [if X = mean % fall in rating scale then Xdrug ÷ Xplacebo = RR].
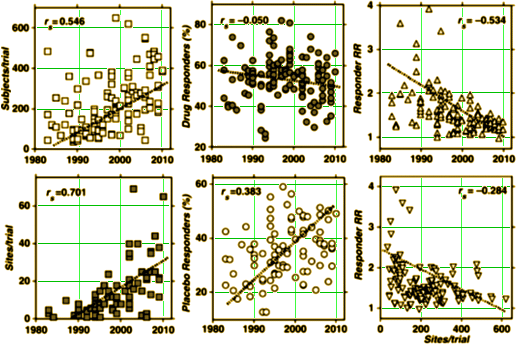
But this paper went further [to the authors’ credit]. They looked at the year the study was done, and the values of Xdrug and Xplacebo over the years [center two graphs]:
I had further separated the Response Rates out by drug class by year:
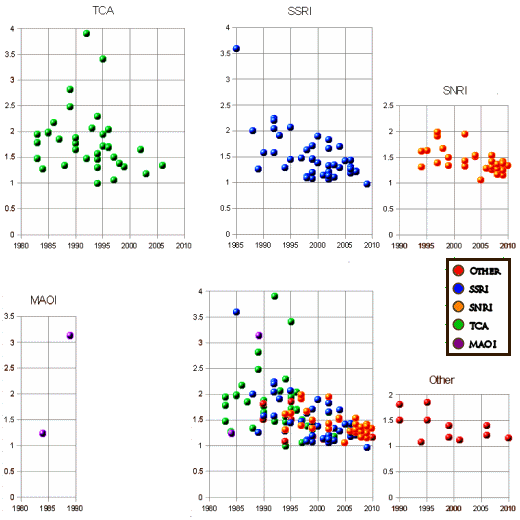
There are several stories there. One is about the deterioration of the quality of clinical trials as they’ve been increasingly privatized and farmed out to the CROs using multiple sites and, I suspect, fewer and fewer patients and more and more recruits. So as much as I hate to admit it, now that I think about it, Trivedi is probably right, it is a ‘virtual world’ [I hasten to add that his studies – TMAP, IMPACT, STAR*D, CO-MED haven’t added much to the knowledge-base about the ‘real world’ either]. But another story is that things have certainly not improved with time, and all the creative novel stuff hasn’t added much either – sequencing, doubling up, augmenting, etc. And I’m betting against genomic directed personalized drug picking too [iSPOT, EMBARC]. Antidepressant medications seem to have a roughly fixed efficacy. No great shakes, but not nothing. If they’d reserve them for Melancholics instead of the virtual disease MDD, I expect they would look a whole lot better.
Why no MAOI’s in the study? I could never understand that. Especially now that selegiline has a transdermal option which partially allows for easing of dietary restrictions and risk.
What I would like to see is a similar animal for every drug used off label and/or adjunctively (the antipsychotics and antiseizure meds primarily).
And lastly, I want a meta analysis on iatrogenic morbidity and mortality from all types of treatment: chemical, electrical, surgical, psychological and environmental. Pick the top ten billed codes for a starter.
Dr. Mickey, you are a statistical visualization guru. Edward Tufte would be proud.
Read his comment yesterday. Having been aware of people who were labelled “Pharmaco Casualities” in college, I’ve suspected as much for a long time. Also, an acquaintance once told me about her experience with a trial for an antidepressant— she said it was obvious who had the placebos and who had taken the antidepressant because there were people walking into walls and having a host of other effects and people who were just sitting around. She got a placebo, and the experience she had watching the people who weren’t so fortunate inspired her to warn everybody she knew in the slightest that it wasn’t worth the money.
We do NEED trials, but they need to be done well.
For those of us who absolutely NEED medication, I think long-term studies should be the norm for drugs and combinations of drugs for maintenance ; and those tests should be done on subjects that surely suffer from what the drug is supposed to treat. By being thorough when recruiting and choosing test subjects, research scientists might also learn more about the mental illnesses they want to treat, to help psychiatrists better identify mental illnesses and treat them. By spending more on studies and less on greasing palms we could maybe see some real breakthroughs from Big Pharma. Or we could just keep suing them and put that money toward independent research.