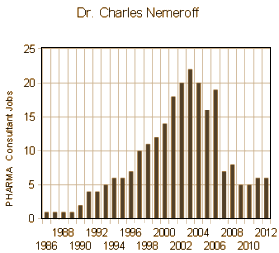
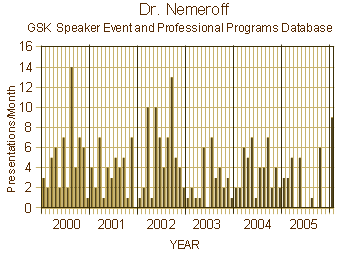
The story that lead GSK to the settlement table paying a $3 B fine for past sins is a long and winding road, a lot of it having to do with the Paxil antidepressant and the Department I was affiliated with at Emory. But even practicing a mile or so from the University, I only heard snippets along the way. I knew the Chairman, Dr. Nemeroff, talked about Paxil, and that one of his researchers, Dr. Zach Stowe, advocated its use in pregnancy. But I was never much of a prescriber and never used Paxil because I found out about its difficult withdrawal syndrome early on – one of those situations where the treatment can be worse than the disease. We now know much more of the story – for example that Dr. Nemeroff was picked by STI’s Sally Laden to moderate Paxil’s launch meeting in 1993 and was on their Advisory Board for the duration. As it turned out, Dr. Nemeroff never met a pharmaceutical company that he didn’t like [left, below], but GSK was always high on the list and ultimately part of his downfall at Emory because of a remarkable number of paid speaker bureau presentations that were unreported [right, below].
… Universities, too, can benefit from the fame and money the deals can bring — a point Dr. Nemeroff made in a May 2000 letter stamped “confidential” that he sent to the dean of Emory’s medical school. The letter, which was part of a record from a Congressional hearing, addressed Dr. Nemeroff’s membership on a dozen corporate advisory boards [some of the companies’ names have since changed].
“Surely you remember that Smith-Kline Beecham Pharmaceuticals donated an endowed chair to the department and that there is some reasonable likelihood that Janssen Pharmaceuticals will do so as well,” he wrote. “In addition, Wyeth-Ayerst Pharmaceuticals has funded a Research Career Development Award program in the department, and I have asked both AstraZeneca Pharmaceuticals and Bristol-Meyers [sic] Squibb to do the same. Part of the rationale for their funding our faculty in such a manner would be my service on these boards.”… For all his fame in the world of psychiatry, Dr. Nemeroff has faced ethics troubles before. In 2006, he blamed a clerical mix-up for his failing to disclose that he and his co-authors had financial ties to Cyberonics, the maker of a controversial device that they reviewed favorably in a journal he edited… The Cyberonics paper led to a bitter e-mail exchange between Dr. Nemeroff and Claudia R. Adkison, an associate dean at Emory, according to Congressional records. Dr. Adkison noted that Cyberonics had not only paid Dr. Nemeroff and his co-authors but had also given an unrestricted educational grant to Dr. Nemeroff’s department. “I can’t believe that anyone in the public or in academia would believe anything except that this paper was a piece of paid marketing,” Dr. Adkison wrote on July 20, 2006…
Notice in the graphs, things changed in 2006 with the loss of his editorship. Emory clamped down. It took watchdogs from inside and outside the profession, inside and outside of Emory, to sound the alarm, and finally an intervention from the US Senate [Senator Grassley] to put a stop to his behavior in 2008. Soon afterwards, Emory finally made some obviously needed policy changes [May 2009]:
| Emory University School of Medicine has a conflict of interest policy addressing the following topics:
|
| Gifts: The acceptance of gifts, regardless of the nature, purpose, or value, from industry and from Emory or faculty start-up companies by individual School of Medicine faculty, staff, trainees, and students is prohibited, on and off the Emory campus. |
| Meals: School of Medicine faculty, staff, trainees, students, departments, and administrative units may not solicit or accept food and drink from industry representatives for School of Medicine activities at Emory or away from Emory. |
| Samples: Individual School of Medicine faculty, staff, students, and trainees may not accept medications and pharmaceutical samples from industry. Gifts of medications and pharmaceutical samples for clinical use will be centrally managed by Emory Healthcare. |
| Vendor Access: Industry representatives are allowed on the premises by invitation only and must register upon arrival. Industry representatives are not permitted to solicit faculty, staff, students, and trainees for sales, marketing, and promotional purposes. |
| CME: The current ACCME-compliant School of Medicine policies on CME are available at http://www.med.emory.edu/CME/index.htm. |
| Scholarships: All gifts of funds from industry to support non-ACCME-accredited education, other educational initiatives, and other professional activities in the School of Medicine must be given centrally through the School of Medicine, and not given to individuals. |
| Ghostwriting: School of Medicine faculty, staff, students, and trainees must not allow their professional presentations and publications of any kind, written or oral, to be prepared by a ghostwriter, whether from industry or otherwise. |
| Speakers Bureaus: Participation as presenters in Speakers Bureaus is prohibited for School of Medicine faculty, staff, students, and trainees. |
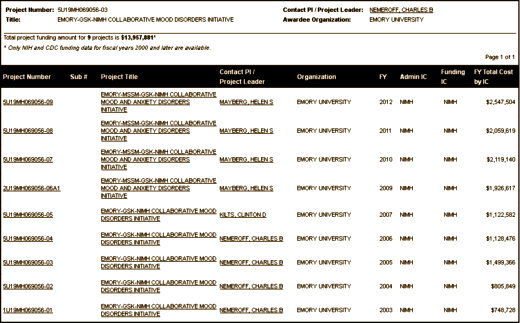
Another piece of the Nemeroff/GSK alliance was also impressive – a $14 M [and counting] Emory/GSK/NIMH Collaborative initiative [that has essentially produced nothing]. Notice the changes in P.I. in response to the Nemeroff revelations:
 Nemeroff was not alone. There were others who bartered their prominance and the reputation of their university on this same exchange. But there were two that I’ve come to think of as specifically in league with him, Martin Keller of Brown University and Alan Schatzberg from Stanford, even though we don’t know as much detail about their careers. The things that connect them in my mind? They were new chairmen together around 1990; they were working
Nemeroff was not alone. There were others who bartered their prominance and the reputation of their university on this same exchange. But there were two that I’ve come to think of as specifically in league with him, Martin Keller of Brown University and Alan Schatzberg from Stanford, even though we don’t know as much detail about their careers. The things that connect them in my mind? They were new chairmen together around 1990; they were working for with GSK and ghost-writer Sally Laden of STI; they were always front and center on pharma issues; they wrote, presented, and played together; they were resume builders moving from neuro-fad to neuro-fad; and they were similarly ethically challenged, making it to Senator Grassley’s list together for unreported personal pharma income. I started thinking of them as a cartel when I read …
Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States.
by Nemeroff CB, Kalali A, Keller MB, Charney DS, Lenderts SE, Cascade EF, Stephenson H, and Schatzberg AF.
Archives of General Psychiatry. 2007 64[4]:466-72.
But they landed on their feet. Dr. Nemeroff moved on to Miami with the help of Dr. Insel, and he’s getting back up to speed [Renowned Clinician-Researcher Named Chair of the Year]. Keller stayed on at Brown continuing to receive a lot of grant money in spite of constant scrutiny because of the infamous Paxil Study 329. Schatzberg was APA President and stayed in the news with pharma supporting projects even after his GSK financed, ghost-written textbook with Dr. Nemeroff was exposed. All three were investigated by their universities, but came through relatively unscathed. Only Dr. Keller has retired, abruptly this summer shortly after the GSK settlement, shortly after the coming of a new Brown President. While it’s just a guess, the new president didn’t call for a retraction of Study 329 as hoped, but Keller retired right around the time of the settlement, and my fantasy is that it was by invitation. As best I can determine, Brown still has no policy on ghost-writing.
There have been other boys and girls of pharma during these years, but few so dedicated or destructive as these three [whether they were joined at the hip in fact, or only in my speculations because they had similar industry-based formulas for success]. They brought lots of money to their departments. They were everywhere: organizations, political positions, Clinical Trials, articles galore, presenting constantly. They were all three smart and up on the latest happenings in the field. They were charismatic and well liked by their staff and students. In spite of this whirlwind of activity, they added little of note to the scientific record. GSK was a river that ran through each of their stories. And while they have different styles, they are all three bullies – wielding power and lashing out when challenged. Oh yeah, they all got rich being academic psychiatrists – not really possible before this era.
The Mickey Mouse award that Dr. Nemeroff just received in Orlando was bestowed by one of his partners in pseudo-education, an outfit known as CME Outfitters. They were roundly chastised by the national CME accrediting body for running a misleading and noncompliant program that featured Dr. Nemeroff. You can read about it here on Health Care Renewal and in The New York Times. So much for his rehabilitation.
I could see it was Mickey Mouse, but the CME Outfitter web site is covered with certifications. Thanks for the back story. It’s a typical Nemeroff all the way – down to the Miami web page entitled Renowned Clinician-Researcher Named Chair of the Year. Some things just never change…
“Internationally renowned”? Really? And just who were the previous honored academic psych chairs Nemeroff speaks of?
Who were the previous ones? Couldn’t locate them through a web search. Maybe they weren’t ‘internationally renowned” enough.
Oh and now Miami is ranked 29 among psychiatry departments in NIH funding, according to the Miami PR puff piece about his award? Seems a bit wanting for an internationally renowned psychiatric chair! Hell that department wouldn’t even get a BCS bowl bid with that ranking!
As long as a profession, organization, or sanctioned group of legitimate representation either prominently supports or just lays silent while unethical, immoral, or worst, illegal behaviors and actions go unchallenged, any and all involved with said perpetrators are guilty.
Look at Penn State with the Sandusky fallout. Evil thrives when good men lay silent. Umm, except here in psychiatry, who are these alleged good men? Haven’t really seen or heard enough of them to think psychiatry as a whole is responsible and appropriate. Again, did I miss the ego test residents had to pass in patriarchal narcicism where successful applicants scored 2 standard deviations above the norm?
Standard deviations, boy is that term fitting here!
Nemeroff must have a very clever PR firm.
Perhaps GMYR http://gymr.com/ is refurbishing Nemeroff’s image.
From http://www.ahrp.org/cms/content/view/327/149/ “For example, The Executive Summary of ACNP’s Task Force on SSRIs and Suicidal Behavior in Youth, was issued by GYMR, a public relations firm in Washington (in January 2004) 10 days prior to an FDA advisory committee hearing….”