-
The article must look and feel like a scientific study using appropriate logic and format.
-
The article must be based on the genuine data set of the study.
-
The conclusion of the article must confirm the original hypothesis or some acceptable alternative.
- The data set does not actually support a desirable conclusion [or, perhaps, any conclusion], and specifically doesn’t actually support the conclusion of the article.
When the ancient Greeks were formalizing logic, some of them had the idea that one could use the rules of logic to reach absolute truth. Others realized that one could distort logic, so they began to study and categorize fallacy, thinking they might be able to plug the holes and still use logic to arrive at absolute truth. Then, along came the Skeptics who mocked the Dogmatists [the absolute truth guys] and said that relative truth was the best we could do. So, the rigid rules of logic and fallacy didn’t define absolute truth anymore and waited patiently in the dusty logic books until they made a comeback in the programming languages running that light box you’re looking at right now called a computer. We moved on to the new standards of relative truth like "beyond the shadow of a doubt" in a courtroom or the statistical calculations of science – all ways of "weigh[t]ing the evidence."
The kind of ghost-writer we’re talking about, someone like Sally Laden, is able to take the data from a study like Paxil Study 329 which was a negative study by the usual rules of science and work her mojo on it to make it look positive. Although she documented that it didn’t make its primary outcome standard, if she conflated the primary and secondary measures, adding a new parameter that was significant by a fluke, she could conclude it was a significant study. They could rename suicidality as emotional lability and bury it in a list instead of under serious adverse effects. They could rationalize not correcting for multiple comparisons based on grammar. All of these things we refer to as spin, a benign term for lying. So, we all know what kind of ghost-writer we’re pointing at. It’s the kind spoken of in porcine metaphors: "putting lipstick on a pig", "making a silk purse out of a sow’s ear".
Nobody cares if Paula Broadwell’s book about General Petraeus was ghost-written by Washington Post editor Vernon Loeb. It made it more interesting and readable. I doubt many of us care if a graduate student or lesser author writes some guru’s scientific paper as long as they are identified on the by-line. What we care about is when a pro at porcine cosmetics works some mojo on a piece of science and it’s kept a secret. Because if we knew, we’d be more likely to look for the signs that the conclusions in the article are in question. In Paxil Study 329, we had a clue. Sally Laden was mentioned as an editorial assistant in the acknowledgements. But in the paper we’re looking at now, Paxil Study 352, she’s not even mentioned. And we’re not told that three of the authors are GSK employees. And there’s no mention that the other authors had loud conflicts of interest because of financial connections with GSK who funded the study.
We particularly didn’t know that the lead author, Dr. Nemeroff, had his name on a sea of papers in 2001, the year of this paper:
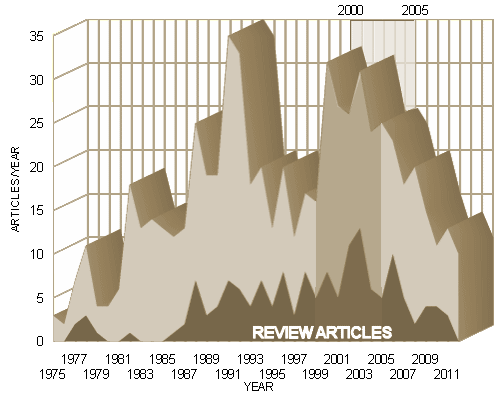
or that in 2001 he worked for 18 pharmaceutical companies including GSK, or that he made 44 paid trips for GSK to give "talks" as part of their Speaker’s Bureau:
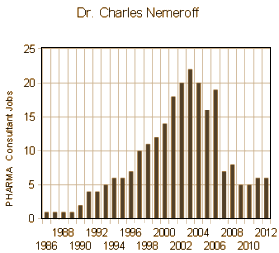
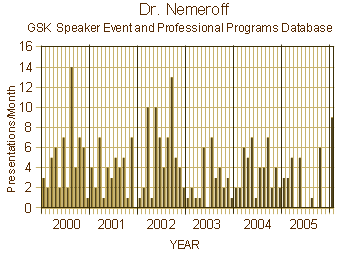
or that he got David Healy un-hired from a position in Toronto for speaking up about PHARMA [before that became cool!…]. Because if we knew any of those things, we might have known to pore over this paper with a fine tooth comb looking for the signs that this was what people call an experimercial, and that the science was likely a lie. But we know those things now, so that’s why we’re looking so belatedly. When a clinical trial article in a medical journal is written by a ghost-writer paid by the industry sponsor of the trial, the bells ring out loudly. Nowadays, they couldn’t even get away with this kind of sleaze.
In the comments of my last post about Paxil Study 352 [paxil study 352 – what’s ghost-writing?], there was a discussion about why I’m harping about an 11 year old paper. We all know Dr. Nemeroff is morally challenged. We all know that Sally Laden was a master manipulator of scientific data. We all now know why conflicts of interest and financial relationships have to be declared. Why beat a dead horse? One reason is that GSK, STI, Sally Laden, the American Journal of Psychiatry, and Dr. Nemeroff continue to pretend this is a legitimate scientific contribution to the psychiatric literature and that its conclusions are valid – Nemeroff even denies it was ghost-written ["we wrote the paper"]. Another reason is that I’m personally ashamed of what happened here and elsewhere in my profession and want us to make it right rather than continue to play like this kind of deceit wasn’t so widespread as to actually characterize our literature of the period. But my most pressing motive right now is that I’m not sure that simply plugging the ghost-writing and conflict of interest leaks is even enough to stop it from happening again. A lot of other people have the same concern.
http://www.pharmalot.com/2012/11/what-will-the-white-house-do-to-the-sunshine-rules/
“There are a lot of complicated ramifications to doing that – things for people in charge to wrestle with. ”
The people in charge in professional societies want to water this down. So they will wrestle with that only in order to kill it. That was my point with the post contrasting Thacker’s statement and AACAP’s statement about disclosure.
If you, or others like you, do not galvanize the bystander clinicians in these societies you won’t have to worry about making nice about anything since nothing will improve.
1BOM,
I apologize for the tone of that last comment. It’s just that I really hope for something to be put forward that any scientist or clinician, even those not steeped in the history of all this (the vast majority), could come to your site and read and say: Well that makes sense to do, there is no major drawback. And then question their respective societies when that leadership tries to oppose it. To burn down these straw men that keep getting used to prevent real change. Scientists and clinicians as a whole seemed turned off by zealotry (even when it’s justified), sadly, which is part of why Stahl came up with the whole pharmascold idea. The kind of disclosures that Thacker and BMJ are talking about could make some real world differences. The evidence is already sufficient to make the case, at least to a large extent. To me the question is now how to help people to want to hear the case and see what they can do to shift the organizations they are a part of. So that there are some organized voices from within the professions trying to push for what Thacker and BMJ are advocating for.
Structured in way they don’t have to be a Don Q and make a career of it. Organized science does not look kindly in these activities: administration, research, clinical, industry, you name it.
To me, rather than simply create parallel organizations like the NPA, professional organization minority voices near to organize AND THEN reach out in an organized way to outside groups to leverage structural change within entities such as The AMA, APA, AACAP, and ACNP.
There is a parallel to union organizing here. Unions will provide tools/resources (and external pressure) to allied voices within non-unionized workplaces. A place like the Safra center at Havard could do this for scientists and clinicians within relevant organizations in psychiatry. This makes it safer for those within organizations to explore acting (again union analogy). Importantly, it recruits experts to look at by-laws and make people aware of what will and allow for real shifts in power. People leave places like AMA or APA because at a certain point they really won’t be allowed to have their voice heard. And, not necessarily because a majority of membership won’t allow it but by those who hold power within the organization. Those organization by laws can be changed.
Again, I apologize for my earlier tone since what I am suggesting is not part of your mission. And you are on a mission. That is part of your strength. I respect Carlat, Healy, Goldacre, but they are still in process of creating their personal brands. You’re on a quest to understand what has befallen your field.
Good luck continuing to be “boring.”
I hope that others out there may lower the price of admission for the bystanders who tire of the status quo. A status quo not doing sufficient justice to this:
““The core of our professional faith, as Spengler points out, is to first do not harm. It harms patients to have biased and corrupted research published. It harms patients to have unaccountable special interests permeate medical research. It harms patients when poor publication practices become business as usual.”
For example see this:
“CMS is Not Authorized by Statute to Expand Reporting to Indirect Transfers (Not Otherwise Specified in Statute) Such as Certified CME”
section of the AMA letter.
http://freepdfhosting.com/eb8ce1742e.pdf
Courtesy of the pharmalot link above.
With friends like these ….
How much money was funneled to Nemeroff through certified CME. Is the AMA really speaking for the majority of its members on that point?
It is hard to imagine that organizational voice changing without structural changes in the organization.
Someone needs to provide tools for that to happen.
Am I misreading that section and its implications on keeping third party payments opaque?
From Pharmalot:
“For instance, the American Medical Association wrote a letter last month to ask the CMS to reconsider having the Center for Program Integrity implement the Sunshine Act “because it will cause significant confusion about the purpose of the transparency reports and create a strong perception that anything contained in a transparency report presumptively raises ethical, fraud, abuse, and program integrity concerns” (here is the letter).
Sources say lobbying has already begun at the White House and the OMB’s Office of Information and Regulatory Affairs to make changes. And one source notes that last year, the same office was behind a decision to gut a key provision contained in financial conflict of interest rules that were being developed by the National Institutes of Health. The provision would have required universities to disclose financial ties between academic researchers and industry on publicly accessible web sites (back story).”
http://www.pharmalot.com/2012/11/what-will-the-white-house-do-to-the-sunshine-rules/
To repost this related comment:
http://www.aacap.org/cs/56th_annual_meeting/
“AACAP has been a leader in managing disclosures and potential conflicts of interest that can and do interfere with objective conduct of science and clinical practice….We are now pleased to give you an update on the Principles, which meet or exceed the standards set by other organizations….While these Principles may prove to be inconvenient, at times, they are serving us well in terms of maintaining our scientific and clinical integrity. We hope you will take pride in our Principles and your participation in the process.”
Followed further down the page by:
“The reporting timeframe is a minimum of the past two years and imminent support. An obligation to report may exist for funds received prior to the last two years if it would be commonly perceived to have impact on that particular presentation, e.g., funding for a study which ended prior to two years ago but is now being reported must be disclosed.”
So, the only hard rule is 2 years and it does not require actual relevant dollar amounts. The slide is here: http://www.aacap.org/galleries/AnnualMeeting/Conflict%20of%20Affiliation%20Slide.ppt
Now see this:
http://www.pharmalot.com/2012/11/one-stop-shopping-proposed-for-conflict-disclosure/
“In a bid to simplify increasing demands to report conflicts of interest held by physicians and researchers, a leading group of academics, policymakers and ethics experts are proposing the creation of a centralized data repository to house all disclosures and increase transparency in medical research and practice.”
“And Thacker cautions that industry lobbying will attempt to influence the final outcome. “My fear is that they’ll go for the most watered-down version and only require disclosing conflicts in the last two years, or not require actual relevant dollar amounts,” he says.”
So:
“My fear is that they’ll go for the most watered-down version and only require disclosing conflicts in the last two years, or not require actual relevant dollar amounts,” – Paul Thacker
“We hope you will take pride in our Principles and your participation in the process.” – AACAP
How much of the hundreds of thousands of dollars in payments to child psychiatrists, adult psychiatrists, and spine surgeons uncovered by Thacker and Grassley would have been shielded under the AMA exception above?
Anyone out there with enough access to break that down?
annonymous, in an another thread (which I’ve only just read), you asked about documentation of Post-SSRI Sexual Disorder(PSSD) — sexual dysfunction that persists after discontinuation.
There are a few dozen papers based on case reports:
Bahrick, Audrey S., and Mark M. Harris, “Sexual Side Effects of Antidepressant Medications: An Informed Consent Accountability Gap.” Journal Of Contemporary Psychotherapy, Vol 39(2), June 2009, pp 135-143.
Farnsworth K, Dinsmore W. Persistent sexual dysfunction in genitourinary medicine clinic attendees induced by selective serotonin reuptake inhibitors. International Journal of STD & AIDS [serial online]. 2009;20(1):68-69.
Csoka AB, Bahrick A, Mehtonen O. Persistent sexual dysfunction after discontinuation of selective serotonin reuptake inhibitors. Journal of Sexual Medicine [serial online]. January 2008;5(1):227-233.
Kauffman, R., Murdock A. “Prolonged Post-Treatment Genital Anesthesia and Sexual Dysfunction Following Discontinuation of Citalopram and the Atypical Antidepressant Nefazodone.” The Open Women Health Journal, 2007 (1), 1-3.
Bahrick, Audrey S, “Post-SSRI Sexual Dysfunction.” ASAP Tablet, Vol 7(3), Sept 2006, pg 2.
Csoka AB, Shipko S. “Persistent sexual side effects after SSRI discontinuation.” Psychother Psychosom. 2006;75(3):187-8.
Text for the above can be found at http://tinyurl.com/6gmblkf
Also see 37 citations here http://www.nationmaster.com/encyclopedia/Post-SSRI-Sexual-Dysfunction
Bolton J, Sareen J, Reiss J. Genital anaesthesia persisting six years after sertraline discontinuation. Journal of Sex & Marital Therapy [serial online]. July 2006;32(4):327-330.
Along with thousands of posts all over the Web, including on http://survivingantidepressants.org, there is a Yahoo discussion group, SSRIsex, with thousands of members.
Some cases of PSSD gradually remit over months or years, but others continue at maximal severity. From what I’ve seen, the worst cases on SSRIsex are men who started on psychiatric drugs when they were children.
There is no doubt this phenomenon exists and, like all other psychiatric drug withdrawal syndromes, is being studiously ignored by clinicians and researchers.
(Paxil is, of course, among the top offenders for PSSD.)
Altostrata,
Thank you.