hat tip to assertTrue() 

I spent the last couple of days in Atlanta seeing old friends, going to some events. After driving back in the rain, I was tired and had an evening watching British videos [Tolkein’s Hobbit and two Ben Goldacres – equally entertaining]. While there, I spent some time with some old psychiatrist friends and had the usual "What happened? How did it get to be this way?" conversations [if you write a blog, people think you’re an expert]. This morning, I was thinking about the whole Clinical Trials thing when I came downstairs and read this comment. I followed the links to this site and ultimately to this 2002 article:
Placebo Response in Studies of Major Depression
Variable, Substantial, and Growing
by B. Timothy Walsh, MD; Stuart N. Seidman, MD; Robyn Sysko, BA; and Madelyn Gould, PhD
JAMA. 2002 287[14]:1840-1847.
[full text on-line]
Objective: To determine whether the characteristics of placebo control groups in antidepressant trials have changed over time.Data Sources and Study Selection: We searched MEDLINE and PsychLit for all controlled trials published in English between January 1981 and December 2000 in which adult outpatients with MDD were randomly assigned to receive medication or placebo. Seventy-five trials met our criteria for inclusion.Data Extraction: Data were extracted from the articles by 2 of the authors and discrepancies were resolved via discussion and additional review by a third author.Data Synthesis: The mean (SD) proportion of patients in the placebo group who responded was 29.7% (8.3%) (range, 12.5%-51.8%). Most studies examined more than a single active medication, and, in the active medication group with the greatest response, the mean (SD) proportion of patients responding was 50.1% (9.0%) (range, 31.6%-70.4%). Both the proportion of patients responding to placebo and the proportion responding to medication were significantly positively correlated with the year of publication (for placebo: n = 75; r = 0.45; 95% confidence interval [CI], 0.25-0.61; P<.001; for medication: n = 75; r = 0.26; 95% CI, 0.03-0.46; P = .02). The association between year of publication and response rate was more statistically robust for placebo than medication.Conclusions: The response to placebo in published trials of antidepressant medication for MDD is highly variable and often substantial and has increased significantly in recent years, as has the response to medication. These observations support the view that the inclusion of a placebo group has major scientific importance in trials of new antidepressant medications and indicate that efforts should continue to minimize the risks of such studies so that they may be conducted in an ethically acceptable manner.
I thought this was a fine piece of work – simple, clean, and a message in a bottle from the time of my own career. I thought the graph was kind of busy, so I deconstructed it:
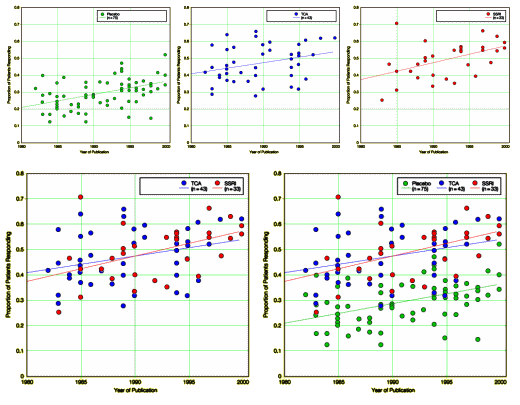
Across the top: Placebo, Tricyclic, and SSRI respectively left to right. Bottom left, Tricyclic against SSRI. Bottom right, all three reunited. I had several thoughts about this data:
-
The ordinate scale is Proportion of Patients Responding [response equal "at least 50% in their score on the Hamilton Rating Scale for Depression and/or a Clinical Global Impression rating of markedly or moderately improved"]. The difference between Placebo and Drug over the two decades is constant at ~0.2 [or 20%]. That gives a Number Needed to Treat [NNT] of 5 – you have to treat 5 patients to get 1 patient who beats the Placebo. That is totally compatible with my own experience [recall that’s Response, not Remission]. People like to say ~ a third, but I think that’s an overestimate. 1 in 5 feels right.
-
There’s no difference between Tricyclics and SSRIs [except adverse effects]. That’s also compatible with my own experience which spans about 40 years [I’d add, there’s little to no difference among SSRIs except adverse effects].
-
What about the creep in placebo effect?
• says commenter Kas Thomas:The drug response has crept up at the same rate as placebo response, which tells me one is the same as the other (one is an "enhanced placebo response" and one is placebo response). Possibly. In any case, when you enroll people who are desperate for free medical care into a trial and pay them money to stay in it, it seems such people are only too glad to tell researchers whatever they want to hear, basically; so it’s no wonder placebo response has crept upward and upward.• say the authors:These observations support the view that the inclusion of a placebo group has major scientific importance in trials of new antidepressant medications and indicate that efforts should continue to minimize the risks of such studies so that they may be conducted in an ethically acceptable manner.• says 1boringoldman:This is the period of the rise of the Clinical Research Industry and I would see it as a transition from academic to industry run studies, from patients to recruits [see beyond approval…]. I’m not totally sure of my timeline here but the idea seems worth considering. This is also the era of the epidemic of research hanky-panky.
Over the last twenty-five or thirty years, we’ve gone nowhere in the psychopharmacologic treatment of depression [other than swapping around adverse effects]. That’s also compatible with my experience…

Sorry, the comment form is closed at this time.