Scorecard Shows U.S. Medical Schools Continue to Make Progress in Pharmaceutical Conflict-of-Interest Policies
POSTSCRxIPT
by Community Catalyst
April 24, 2013The American Medical Student Association released its 2013 PharmFree Scorecard this month, continuing to pressure and encourage medical schools to improve their policies on conflicts of interest and interactions with industry. These policies have impacts on students’ medical education, the future of the medical profession and the care physicians provide. As patients we should be able to trust that decisions about our care are based on science and our best interests, not the marketing strategies of the pharmaceutical industry…
Highlights of the 2013 AMSA Scorecard:
… The 2013 AMSA PharmFree Scorecard, the NPA National Grand Rounds and the Community Catalyst Policy Manual are made possible by a grant from the state Attorney General Consumer and Prescriber Education Grant Program, which is funded by the multi-state settlement of consumer fraud claims regarding the marketing of the prescription drug Neurontin. Partners in the PACME project are AMSA, Community Catalyst, the National Physician’s Alliance, and the Pew Charitable Trusts.
Posted on Wednesday 24 April 2013
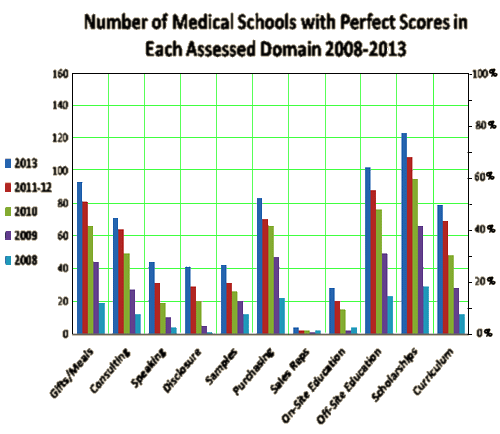
After a long darkness, it seems like we’re getting a few things going the right way lately – successful lawsuits against Pharma’s improprieties, the AllTrials campaign, a growing awareness about the DSM-5 misadventures, more and more encouraging chatter, etc. Here’s some that really feels good – the results from this year’s American Medical Student Association [AMSA] scorecard. For the last 6 years, the organization has been scoring all 158 American Medical Schools based of their policies concerning Conflicts of Interest and their relationships with Industry:
Any way you look at it, this is a remarkable achievement made all the more impressive by the fact that the changes reflect those made by Medical Students with a very simple intervention – a published survey. It portends an enlightened future generation of doctors and once again reinforces the ending line from Ben Goldacre’s TED Talk, "Sunlight is the best disinfectant."
hat tip to Hooked: Ethics, Medicine, and Pharma… 
Sorry, the comment form is closed at this time.
This is encouraging news.
A few years back the talk was about free pens and coffee mugs…
I hope we continue to see a focus on consulting, speaking and research grants – the areas where the big bucks are.
Duane
The damage is already — the faculty is indoctrinated by filet mignon of years past. It will take a die-off for the sludge to be cleaned out.
I’m thinking that exactly ONE researcher being held criminally liable for the damages that a drug does to patients would be sufficient to bring the whole machine to a screaming halt.