Author affiliations:
Poliklinika Neuron, Croatian Institute for Brain Research, Medical School, University of Zagreb, Zagreb, Croatia [Dr Henigsberg]; Takeda Global Research and Development Center, Deerfield, Illinois [Drs Mahableshwarkar and Chen and Ms Jacobsen]; and Department of Psychiatry, University of Pennsylvania, Philadelphia [Dr Thase].
Potential conflicts of interest:
Dr Henigsberg has participated in clinical trials supported by Takeda and Lundbeck. Drs Mahableshwarkar and Chen and and Ms Jacobsen are employees of Takeda. Dr Thase has been a consultant/advisor for Alkermes, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Dey, Forest, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, Lundbeck, MedAvante, Merck, Neuronetics, Novartis, Otsuka, Ortho-McNeil, Pamlab, Pfizer, PGx Health, Shire, Supernus, Takeda, and Transcept; has received grant support from the Agency for Healthcare Research and Quality, Eli Lilly, Forest, GlaxoSmithKline, National Institute of Mental Health, Otsuka, and Sepracor; has participated as a speaker for AstraZeneca, Bristol-Myers Squibb, Dey, Eli Lilly, Merck, and Pfizer; has equity holdings in MedAvante; and receives royalties from the American Psychiatric Foundation [Guilford Publications].
Funding/support:
This study was supported by the Takeda Pharmaceutical Company, Ltd, as part of a joint clinical development program with H. Lundbeck A/S. Assistance with writing and manuscript preparation was provided by Sara Sarkey, PhD, an employee of the Takeda Pharmaceutical Company.
A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder.
by Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, and Thase ME.
Journal of Clinical Psychiatry. 2012 73[7]:953-959.
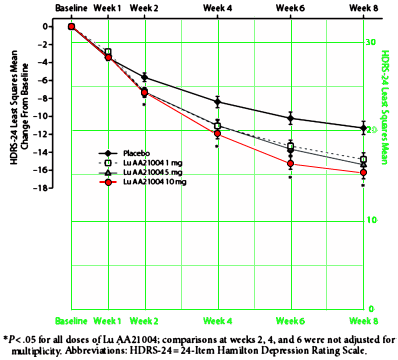
OBJECTIVE: Lu AA21004 is an investigational multimodal antidepressant. This randomized controlled trial evaluated the efficacy and tolerability of multiple doses of Lu AA21004 versus placebo in adults with major depressive disorder [MDD].METHOD: Adults diagnosed with MDD [based on DSM-IV-TR criteria] with a Montgomery-Asberg Depression Rating Scale [MADRS] score ≥ 26 were randomly assigned [1:1:1:1] to receive Lu AA21004 1 mg, 5 mg, or 10 mg or placebo for 8 weeks [between August 2008 and August 2009]. The primary endpoint was reduction in 24-Item Hamilton Depression Rating Scale [HDRS-24] total score after 8 weeks of treatment compared with placebo for Lu AA21004 10 mg. Additional outcomes included response and remission rates, Sheehan Disability Scale [SDS], Clinical Global Impressions-Global Improvement scale [CGI-I], MADRS total score, and HDRS-24 total score in subjects with baseline Hamilton Anxiety Rating Scale [HARS] score ≥ 20. Adverse events were assessed throughout the study.RESULTS: A total of 560 subjects [mean age = 46.4 years] were randomized. There was a statistically significant reduction from baseline in HDRS-24 total score at week 8 for Lu AA21004 10 mg vs placebo [P < .001]. There were improvements [nominal P values < .05 with no adjustment for multiplicity] in HDRS-24 total score, response and remission rates, CGI-I score, MADRS total score, and HDRS-24 total score in subjects with baseline HARS score ≥ 20 at week 8 for all Lu AA21004 treatment groups vs placebo. No significant differences were seen in SDS scores between any dose of Lu AA21004 and placebo. The most common adverse events were nausea, headache, and dizziness.CONCLUSIONS: After 8 weeks of treatment with Lu AA21004 10 mg, there was a significant reduction in HDRS-24 total score compared with placebo in adults with MDD. Lu AA21004 was well tolerated in this study.ClinicalTrials.gov identifier: NCT00735709.
Investigative Depression Drug Does Better in Europe than United States
Medscape News
by Deborah Brauser
May 18, 2011"The multidose study gives us reason to think that this particular compound may indeed have value and there may be a range of doses that are valuable," investigator Michael Thase, MD, professor of psychiatry at the University of Pennsylvania School of Medicine in Philadelphia, told Medscape Medical News. He reported that before conducting that study, the investigators speculated that the 5- and 10-mg doses of Lu AA21004 would do well but were pleasantly surprised when the 1-mg dose was effective also.
As for the second study, Dr. Thase said he’s not sure if it failed because of the dose assessed "or because many studies fail in the US right now. "That study humbles us because even half the studies of drugs that we know work have failed to show they work in the United States. And this is partly because the placebo response has been growing dramatically over the last 30 years."
He said placebo response rate was around 20% in 1970 and is about 45% now. "Anytime the placebo response in a depression study goes over 40%, the chance of the study failing goes well over 50%. And the US study did have a high placebo response rate," said Dr. Thase. "Although we won’t know more until there are 3 or 4 or 5 more studies, our [multicountry] trial gives us reason to believe we’re on the right track with this multiaction agent. And the US study tells us it’s just not easy to demonstrate that an antidepressant works under these circumstances"…
Why even bother to vet a 4 year old study presented two APAs ago? It’s time for a change in the clinical trials for psychiatric medications. The presentation of these industry studies is so uniformly jury-rigged that they ought to be banned from our peer reviewed literature. Attempts at reform like clinicaltrials.gov have failed because the companies just don’t post the results. There’s now a move afoot to insist that they publish their raw data [AllTrials] which would be the only way to really vet the studies instead of just pick at them as I have done here and in academic?…. And this practice of pulling in a quaisi-academic KOL who is as experience distant from the study as the reader is just inappropriate theatrics.
Dr. Thase’s comments about the placebo effect, particularly in the US, are delivered with irritation [at who knows what]. In my way of thinking, it can only reflect that the study subjects weren’t particularly ill in the first place – a recruitment issue that says something about the whole CRO enterprise. But back to why vet these studies. Right now, the FDA is considering this drug for approval based on four later studies that are yet unpublished and have no results posted on clinicaltrials.gov [way past time…]. They are industry funded, likely CRO run studies just like these. They were presented at the 2013 APA just like these. They were discussed by an apparently uninvolved quaisi-academic KOL just like these.
Two psychological factors of the test subjects I’m wondering about— relief at finishing the trial and the reward of being paid.
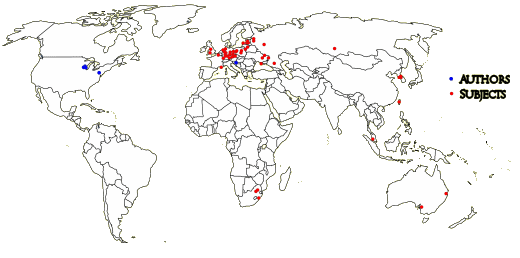
Your red dots, dr Nardo, are dense in Eastern parts of Europe, in the Baltic countries and a few in Russia. They share the characteristics of former Soviet dominance, brutal transitions to market economy, inequality, enclaves of povertystricken people – long since on the map of clinical trials organizations-companies as good for business. Quicker enrollment, cheaper subjects, less interference from public oversight authorities, less risk of being held to account for any adverse consequences. As planned and as advertised in Fierce Pharma by some of these companies. No trials in India, perhaps as the Indian government is more on the alert to irresponsible activities, sickness and death in the wake of Big Pharma and their CROs. No trial in Denmark, home to Lundbeck, none in Sweden, Finland or Norway…
does the author-site map represent the shape of things to come?
sara sarkey phd linkedin profile
http://www.linkedin.com/pub/sara-sarkey/21/581/843
Sara Sarkey
Publications Manager at Takeda Pharmaceuticals
Greater Chicago Area
Pharmaceuticals
Previous
Takeda Pharmaceuticals North America,
Takeda Pharmaceuticals,
Purdue University Calumet
For some added context within which one is trying to “do something about it”:
http://www.pharmalive.com/valeant-bausch-lomb-and-the-long-reach-of-fred-hassan
Clinical trails GPS:
http://www.clinicaltrialsgps.com/
People,
It seems that the denunciation of psychiatry is going mainstream,
http://mobile.nytimes.com/2013/05/28/opinion/brooks-heroes-of-uncertainty.html
“The problem is that the behavorial sciences like psychiatry are not really sciences; they are semi-sciences. The underlying reality they describe is just not as regularized as the underlying reality of, say, a solar system.”
“If the authors of the psychiatry manual want to invent a new disease, they should put Physics Envy in their handbook. The desire to be more like the hard sciences has distorted economics, education, political science, psychiatry and other behavioral fields. It’s led practitioners to claim more knowledge than they can possibly have. It’s devalued a certain sort of hybrid mentality that is better suited to these realms, the mentality that has one foot in the world of science and one in the liberal arts, that involves bringing multiple vantage points to human behavior.”
EXACTLY.
…”it’s time to stop just decrying this stuff and do something about it.”
It’s the responsibility of national governments to protect the citizens against fraudulent health care products and procedures that do more harm than good to individuals, families and society. Regulation. Politics.
While awaiting serious action from governments, we can get serious and educate ourselves and those we love and know to the inherent risks in medicine and drugs and get weaned from quick fixes and ideas of life without hurt.
And then, perhaps, we’d have energy to save the world too, from the big predators, corporate, political, drugs and drones
The focus on “doing something about it” needs to move beyond drug makers and researchers. It’s easy to point to corporate greed and those “nasty pharmaceutical companies.”
But the reality is that it is *not* the responsibility of these companies to practice medicine.
Doctors are the ones who are entrusted to know about the drugs they prescribe.
The buck stops with the doctors.
Duane
Some of us have lost faith in medicine and doctors…
We visit the doctor only for emergencies, when all else fails.
Otherwise, we accept full responsibility for our healthcare, without professional guidance.
“If you want something done right, you have to do it yourself. This especially includes your health care.” – Andrew Saul, Ph.D., Editor, Journal of Orthomolecular Medicine –
http://www.doctoryourself.com
Duane
I have been nagged by my editors to chime in on the DSM-5 issue directly, and so I have. I’ll never approach Mickey’s level of performance — he is the Sandy Koufax of the blogosphere — but here is a Toledo Mud Hen rookie’s attempt to nick the strike zone:
http://harvardpress.typepad.com/hup_publicity/2013/05/suffering-and-sadness-are-not-diseases-richard-noll.html
“This time around, we’ll need to look out not just for the research subjects, but also for the rest of us who get the treatments vouched for by that research.”
Johanna Ryan
from:http://davidhealy.org/when-does-yes-mean-no/?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+DrDavidHealy+%28Dr.+David+Healy%29
Here is more evidence, were any needed, that clinical trials in psychiatry have descended to Kabuki theater, in the sense of skilled fakery. The sheer tediousness of these ritualized exercises, compounded by the dissembling analyses, by the corporate propaganda, and by the KOL-uttered boosterism gives the game away.
Psychiatry has been used as a tool to create a very uncivil society, we need psychiaty that observes a social contract:
https://en.wikipedia.org/wiki/John_Locke#Political_theory