Dr. Nemeroff’s presentation in London felt like a postcard from the past – a postcard from the time of Paxil Study 329 and TMAP, the time of ghostwriters and chemical imbalance, the period when academic psychiatry and commercial enterprise wore each other like costumes at Mardi Gras bathed in the warm glow of future discovery just up ahead. it was a time when psychiatry had been freed from the shackles of speculation and transported into the light of a new century.
Yesterday, I watched Dr. Nemeroff’s Grand Rounds presentation at NYU last year again. It was the part at the end about treatment implications that got to me. That story starts with a 2000 industry-funded non-placebo controlled comparison of a forgotten antidepressant and Cognitive Behavior Therapy or the combination. There had been innumerable earlier studies that showed that CBT plus antidepressant beat either one of them alone – so many that we used to joke that every new researcher must have to do a CBT/Antidepressant study as a rite of passage. What was unique was the army of authors and the fact that the New England Journal didn’t have room for all the industry disclosures. The article launched editor Marcia Angell as an anti-COI activist.
I said forgotten antidepressant because by 2003 when Nemeroff’s paper was published, Nefazodone [Serzone®] was being withdrawn from the market in Europe and stopped being sold in the US in 2004 because of hepatotoxicity. So it’s hard for me to see that 2000 study with its cadre of add-on authors as anything but business as usual in an era of junk science, and Dr. Nemeroff’s paper as an afterthought résumé-churner. Speaking of rites of passage, if a post-doc fellow had presented a secondary analysis of an industry funded, non-placebo controlled study to the Institute of Psychiatry at Kings College and made global pronouncements about how to treat chronically depressed patients based on the findings, I think he or she would have been shredded from the floor.
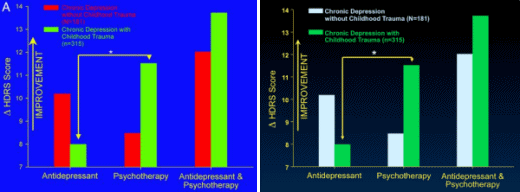
Results of the analyses of variance comparing change in Hamilton Rating Scale for Depression scores as a function of treatment type and early life trauma histories as well as Fig. 1A reflect change relative to the first week of treatment instead of baseline. When change scores relative to baseline are used, the interaction effects between treatment type and childhood trauma histories are not statistically significant. This discrepancy is due to marked changes in depression scores during the first week of treatment.
Note that all analyses comparing the more conservative outcome measure of remission as a function of treatment type and childhood trauma as well as Fig. 1B are correct. Thus, consideration of treatment response relative to baseline does not detect the effect of childhood trauma on final remission, whereas consideration of final response relative to first response does detect the effect.
Dr. Nemeroff is described as charismatic. I don’t know about that, but he’s certainly at home on a podium. All through his career, people have allowed him to get away with things they wouldn’t tolerate in others. They see him as bright, engaging, boyish, maybe an embellisher, but in an innocent sort of way. He’s used to being let off the hook. When he recommended treatments that he had a financial stake in without mentioning it in 2004, he got a hand slap. When he was exposed for writing signing on to a ghost written review of a vagal nerve stimulator with other coinvestors without mentioning his or their stake, he said it was a clerical error and gave up his editorship. When he was busted for unreported income from GSK while PI on a joint GSK NIMH grant and removed as chairman, he said he didn’t understand the rules and got another chairmanship within the year. All of those explanations were untrue, as were his defenses when he was exposed for a ghost-written textbook by POGO a year later.
Re: “This discrepancy is due to marked changes in depression scores during the first week of treatment.”
This seems like a red flag.
Don’t the drug makers say it takes 2-6 weeks to begin to see changes?
Duane
Duane,
That’s a good point. What they mean is it takes 2-6 weeks for them to show the drug separating from placebo. In clinical trials, they call this rapid fall in the early weeks “the placebo effect” because it’s hard to explain. But as for the meaning of their comment, “This discrepancy is due to marked changes in depression scores during the first week of treatment.”, it doesn’t make any sense to me either. I don’t see that on their graph from the original study:
There are lots of red flags in the 2003 PNAS publication by Nemeroff. In particular, it was “communicated” by Wylie Vale. Who is Wylie Vale? In 2003 he was a member of the Institute of Medicine of the National Academy of Sciences. In those days, if you could get a member to sponsor one of your manuscripts you were likely to get published in PNAS. The eminence of the sponsoring member carried a lot of weight. The sponsoring member was supposed to be knowledgeable on the subject. Did Wylie Vale meet that requirement? No way. Wylie Vale was a basic endocrinologist at the Salk Institute in San Diego who discovered corticotrophin releasing hormone (CRH). Wylie Vale didn’t know squat about clinical trials of nefadozone or cognitive behavior therapy. So what was the connection between Wylie Vale and Charles Nemeroff? Nemeroff was in hot pursuit of a CRH theory of depression, possibly linked to child abuse, and he had a financial relationship with Wylie Vale’s company Neurocrine that was developing a CRH antagonist. This looks like an example of good old boy cronyism and backscratching at its worst. Has there really been corruption in the academic-industrial complex? This is exhibit A. One is tempted to guess that the data were so weak that regular journals rejected the report, so Nemeroff did an end run with Wylie Vale’s help to PNAS. He has dined out on it ever since. Was any of that disclosed in the PNAS article? Silly me for asking.
Nemeroff also gave his friend Wylie a payback with this: The neurobiology of depression: inroads to treatment and new drug discovery. Nemeroff CB, Vale WW. J Clin Psychiatry. 2005;66 Suppl 7:5-13. Review. PubMed ID: 16124836.
As things played out, Wylie Vale died suddenly and prematurely a couple of years ago and clinical trials of CRH antagonists in depression went nowhere. So, Nemeroff has only stale old cards to play nowadays – like the retracted data from 2003.
Dr. Carroll,
Thanks for clearing up both the PNAS and CRH questions. The CRH thing always seemed to be aiming at some kind of commercial goal, but I couldn’t figure what it was.
Mickey,
The placebo effect is strong, very “real“.
IMO, we ought to find ways to capitalize on the power of placebo in helping people overcome, recover, get strong… without harm.
Duane
You’re welcome, Mickey… I know where the bodies are buried! LOL.