With the Institute of Medicine [Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk] and the National Institute of Health [Honoring Our Promise: Clinical Trial Data Sharing] joining the call for Data Transparency, we’re beginning to approach the details, wherein dwells the devil – what data? which trials? The whole notion of proprietary ownership of the raw dat from Clinical Trials never made any real scientific sense in the first place [see except where necessary to protect the public…, a crushing setback…, the end game… ] being justified by trade agreements. The main two arguments in this last year against Data Transparency have been protecting Commercially Confidential Information and Patient Confidentiality. But first, yet another review of the landscape:
what data?
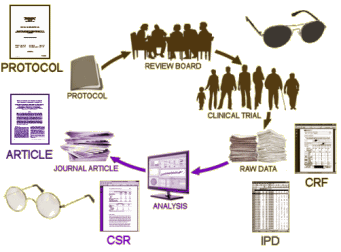
In the discussion that follows, Summary Data refers to the CSR [Clinical Study Report] which does not necessarily or usually contain the raw data. It’s got means, standard deviations, summary tables, etc. But it doesn’t have the individual test scores or the actual clinical observations of adverse events. The IPD [Individual Participant Data] does have the raw numbers from the individual participants, but the clinical observations are in tabular form – transcribed from the CRFs [Case Report Forms] which is as close as one can get to being there. And one mustn’t forget the a priori Protocol – the plan for the study before it commenced.
which Trials?
There seems to be a consensus developing that, going forward, the more comprehensive data [IPDs and CRFs] should be available for independent review to qualified reviewers. It levels the playing field between the sponsors/investigators and the independents. But what about Clinical Trials from the past [called below legacy trials]? Here, Ed Silverman interviews one of the members of the IOM panel on that very point:
Pharmalot: WSJBy Ed SilvermanJanuary 20, 2015Last week, the Institute of Medicine issued an eagerly awaited report about sharing clinical trial data that recommends government agencies and companies provide access from research studies that they fund. The agency suggested timetables for such things as summary results and complete data packages. The move comes after heightened controversy over sharing data, how much data and the best way to do so. Among the issues that remain unsettled is the extent to which data from older trials will be shared. We spoke with Ida Sim, a professor of medicine at the University of California, San Francisco and a member of the IOM committee that prepared the report, about reaching back to past studies. This is an excerpt.
Pharmalot: To what extent did the committee look at sharing data from older trials?
Sim: It is addressed. We did think about it. But there are special considerations for sharing data from legacy trials… The first distinction to be made is between summary level data and individual patient level data. The average result of the trial, which is the summary, is a sort of bottom line – that’s an average result. Advocacy groups like Alltrials are asking for the release of summary level data. But the report focused on some sense of the value of individual patient-level data, and sharing the specific numbers that go into the average is much more complicated, especially for legacy trials.Pharmalot: How so?
Sim: The primary challenge is the issue of informed consent. Patients who participated in trials in the past were most likely in trials that did not include [a provision for] sharing data publicly. So if we want to now share data, ethically, investigators should go back to get informed consent from the participants. There’s another complication. Very often investigators and the staff associated with a trial have scattered. So it can be expensive and challenging to pursue a team of people, maybe years later, to have them pursue this.Pharmalot: Should that preclude all older trials?
Sim: Well, that said, for major significant trials that do influence decisions for clinical care, we recommend that, on a case-by-case basis, legacy studies should be prioritized for data sharing. But we have to be pragmatic and realistic. These are issues that have been going on for a long time. I think the committee has been building on prior work [of others] and recognizing there were instances of cherry picking of results, which started the whole movement of registration disclosure [registering trials with ClinicalTrials.gov]. Now, we’re pushing for the next level. But it’s incremental.Pharmalot: Do you worry that companies may be let off the hook?
Sim: They have been really. The real question is what to extent can we go back and put them on the hook. It’s a question of balance. There are studies from academic investigators, who also have not been upfront [about disclosure]. There are problems of transparency across the whole clinical trial enterprise and the concerns apply to all clinical trials. The ones from the pharmaceutical industry are just more apparent to the public…
But, as Dr. Sim says, those legacy trials are going to contain a lot of jury-rigged science. And as a scientist/physician, I feel betrayed, and I want it exposed in all its gory detail. I, and the rest of medicine, have been actively misinformed – on purpose. Our patients have been actively misinformed – on purpose. And I personally believe that the companies and their medical allies will just do it again if there’s not a truth and reconciliation period to mark what happened in stone. But that’s my opinion, my bias that surely affects my logic supporting full Data Transparency for legacy trials. From my vantage, the operative saying here is, "Don’t do the crime, if you can’t do the time."
But there’s another good very reason for full Data Transparency for legacy trials, particularly in psychiatry. The drugs in question are now off-patent – widely available in generic form, inexpensive, and still in heavy use. Managed Care reviewers still hawk them as cost-cutters. And they’re not going to be replaced any time soon. Nor is it likely that Bill Gates, PHARMA, or the NIMH will finance any new trials to restudy them properly. But we can look at the raw data from the original trials. And fortunately, the sleight of hand occurred primarily in the analytic and publication processes that came after the blinds were broken. So those legacy trials are the very ones that need to be reanalyzed and meta-analyzed by independent investigators playing with a full deck. Without an accurate and very public re-appraisal, the problem is going to be perpetuated for decades.
The whole confidentially issue with legacy trials is a straw man issue, and I am sure said with a straight face. The participants will be nothing more than data points, which in reality, they will be in the summary.
Past history shows how this raw data has been massaged to generate the response pharma wants, not what are the true results of the trial.
The business issue is that pharma is still a player in the generic market often simply creating a new subsidiary to market what is an old product. Things such as Tamiflu will came back to haunt them if data becomes available.
Just as an aside, I have seen Tamiflu commercials on my local television stations.
Steve Lucas
I agree, Steve, but I wonder if the pharma jargon about confidentiality doesn’t confuse and cow legislators.