by March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, and Severe JArchives of General Psychiatry. 2007 64[10]:1132-1143.
CONCLUSIONS: In adolescents with moderate to severe depression, treatment with fluoxetine alone or in combination with CBT accelerates the response. Adding CBT to medication enhances the safety of medication. Taking benefits and harms into account, combined treatment appears superior to either monotherapy as a treatment for major depression in adolescents.
by Benedetto Vitiello, Susan Silva, Paul Rohde, Christopher Kratochvil, Betsy Kennard, Mark Reinecke, Taryn Mayes, Kelly Posner, Diane E. May, and John S. March.Journal of Clinical Psychiatry. 2009 70[5]: 741–747.
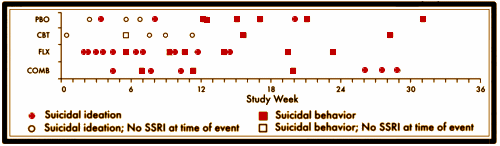
RESULTS: Forty-four patients [10.0%] had at least 1 suicidal event [no suicide occurred]. Events occurred 0.4 to 31.1 weeks [mean +/- SD = 11.9 +/- 8.2] after starting TADS treatment, with no difference in event timing for patients receiving medication versus those not receiving medication. Severity of self-rated pretreatment suicidal ideation [Suicidal Ideation Questionnaire adapted for adolescents score > or = 31] and depressive symptoms [Reynolds Adolescent Depression Scale score > or = 91] predicted occurrence of suicidal events during treatment [P < .05]. Patients with suicidal events were on average still moderately ill prior to the event [mean +/- SD Clinical Global Impressions-Severity of Illness scale score = 4.0 +/- 1.3] and only minimally improved [mean +/- SD Clinical Global Impressions-Improvement scale score = 3.2 +/- 1.1]. Events were not preceded by increased irritability, akathisia, sleep disturbance, or manic signs. Specific interpersonal stressors were identified in 73% of cases [N = 44]. Of the events, 55% [N = 24] resulted in overnight hospitalization.CONCLUSIONS: Most suicidal events occurred in the context of persistent depression and insufficient improvement without evidence of medication-induced behavioral activation as a precursor. Severity of self-rated suicidal ideation and depressive symptoms predicted emergence of suicidality during treatment. Risk for suicidal events did not decrease after the first month of treatment, suggesting the need for careful clinical monitoring for several months after starting treatment.
« truncated » · « recolored » · « click for original »

This disconnect between the narrative and the data came to my attention from the posts by David Healy [Coincidence a fine thing] and Robert Whitaker [The Real Suicide Data from the TADS Study Comes to Light]. I ran across them vetting an absurd paper by statistician, Robert Gibbons [Suicidal Thoughts and Behavior With Antidepressant TreatmentReanalysis of the Randomized Placebo-Controlled Studies of Fluoxetine and Venlafaxine], in one of his many attacks on the Black Box Warnings [a warning that the TADS trial actually supports]. Both Healy and Whitaker [and I] attribute the discovery of this damning chart amid the din of TADS papers to Swedish Investigator Göran Högberg [his comments from our 2012 email conversation are in my significant I… post].
by Högberg, Göran | Antonuccio, David O. | Healy, David.International Journal of Risk & Safety in Medicine. 2015 27[2]:85-91.
Completed suicides are a major cause of death in adolescents in Sweden. Forensic analysis of completed suicides in children and adolescents shows there is one completed suicide per 1000 children taking a selective serotonin re-uptake inhibitor [SSRI]. In order to elucidate these events we undertook a study of the results and reporting of suicidal events in the Treatment of Adolescents with Depression Study [TADS]. We conclude that a major, albeit underreported, finding in the TADS was the significant increase of suicidal events in the adolescents on antidepressant medication in comparison to the group on placebo medication. The proportions of suicidal events were 11% and 2.7% respectively. This increased risk of suicidal events might be related to the high incidence of medication with an SSRI in the group of completed suicides among Swedish adolescents:
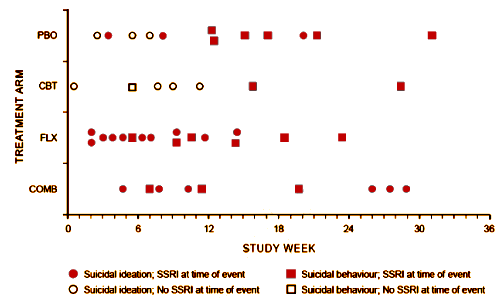
Something I do not understand, maybe someone can explain it. Why are there 2 red bubbles in the placebo group before the 12 week marker? How are those ideation on SSRI if the initial study length was 12 weeks?
I haven’t figured that out either. It’s on every version, and so far I can’t find anything in the text that explains it. I’m going to send that question to the authors.
An eagle-eyed reader found it in Suicidal Events in the Treatment for Adolescents with Depression Study [TADS].
“Some patients who had been randomized to CBT (N=2) or PBO (N=9) and had a suicidal event were in fact on SSRI edication at the time for the event having started antidepressant treatment because of non-response to the randomly assigned treatment. For these patients, tune to event was recomputed by taking the tune when SSRI medication was initiated as the starting point. Using this approach, for all the patients on SSRI at time of the event (N=36). the time to event was 10.0 ± 7.7 weeks (range 0.7—28.9), which was not statistically different from that for the patients not on antidepressant medication at the time of the event (N=8: 5.8 ±3.2 weeks, range: 0.4-10.4; Wilcoxon two-sample test, z=-1.2[ p=0.22).“
I get it now. So aside from being misleading in its original labeling the bottom of the chart also represents two completely different timelines, depending on what group the patient is in. This is like a study in how to design an incomprehensible chart. If you wanted to figure out at what point after initiating treatments someone had a suicidal event, you could do that with a separate chart. This chart seems to be trying to do two things at once and completely messing up both of them as a result. Or maybe the purpose of making a chart like this was to deceive anyway.
In the Suicidal Events TADS study, it could be researchers were looking for obvious “evidence of medication-induced behavioral activation.”
See Hirose, S. (2003). The causes of underdiagnosing akathisia. Schizophrenia bulletin, 29(3), 547-558. Free pdf at http://schizophreniabulletin.oxfordjournals.org/content/29/3/547.short
From what I’ve seen, added to those factors identified by Hirose should be that even adult patients do not have the vocabulary to accurately convey how they feel. The internal sensations of akathisia are not easily described.
Ryan,
Without the raw data, there’s really no way to fix it. I agree that in this case, two charts are better than one.
Thanks for pointing out these important “inconsistencies.” As someone who reviews studies for journal publication, I’ve had to get better at spotting data manipulations and clever attempts to emphasize or de-emphasize certain things. These can appear not only on pharma studies but also from people trying to support new types of therapy. Thanks to the previous commentators who figured out how some patients got SSRI when randomized to placebo. That one vexed me too.
However, I’m not sure this chart is as monumental as described. As I understand the study, the blind was broken after 12 weeks and patients were allowed to switch treatments. This may reflect the study authors’ own bias, but it sounds like the patients whose depression was getting worse on placebo or CBT were started on an SSRI. Putting all the causality here on the medication, then, is a bit dubious. That said, there was better evidence for more suicidality related to SSRI use during the acute 12 week period but, as was mentioned in the post, this was acknowledged in the original TADS publication.
Another interesting phenomenon I’ll point out, is how certain folks seem so convinced SSRIs can have this huge powerful negative effect on the brain while discounting any chance that for some people it really helps. Can’t it be that some are helped, some harmed, and some neither one? Is this not the case for almost all prescription medications?
Another interesting phenomenon I’ll point out, is how certain folks seem so convinced SSRIs can have this huge powerful negative effect on the brain while discounting any chance that for some people it really helps. Can’t it be that some are helped, some harmed, and some neither one? Is this not the case for almost all prescription medications?
I completely agree…