The place of antipsychotics in the treatment of Psychosis remains clouded in controversy in spite of more than a half century of study, experience, and debate. Neither Kraepelin’s Dementia Praecox, a progressive deteriorating illness leading to an early death, nor Bleuler’s Schizophrenia, a defined syndrome with multiple types, survives as the dominant model for predicting the course of illness. We now tend to see episodes of psychosis punctuating a variable level of functional impairment over time. While traditional guidelines call for maintenance medication based on relapse prevention, long term studies document that many patients regularly discontinue the treatment. And others feel strongly that maintenance medication itself interferes with recovery. Both of these opposing recommendations are backed by studies, anecdotal case reports, passionate ideologies, and interpretations of the bias of the opposing view. Since these medication have now been around now for a lifespan we’re seeing large population surveys that bear on this controversy. This one’s from Scandanavia, a traditional resource for this big population studies:
by Torniainen M, Mittendorfer-Rutz E, Tanskanen A, Björkenstam C, Suvisaari J, Alexanderson K, and Tiihonen J.Schizophrenia Bulletin. 2015 41[3]:656-663.
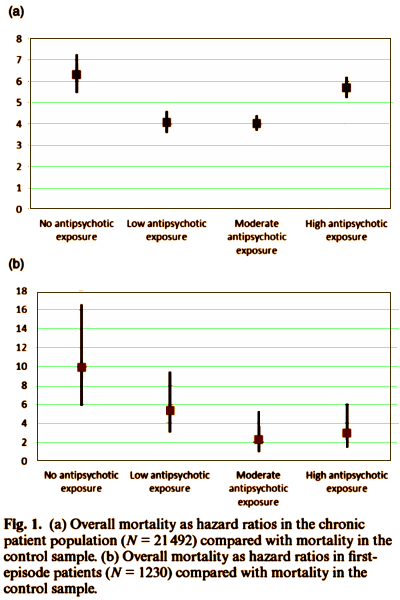
BACKGROUND: It is generally believed that long-term use of antipsychotics increases mortality and, especially, the risk of cardiovascular death. However, there are no solid data to substantiate this view.METHODS: We identified all individuals in Sweden with schizophrenia diagnoses before year 2006 [N = 21 492], aged 17-65 years, and persons with first-episode schizophrenia during the follow-up 2006-2010 [N = 1230]. Patient information was prospectively collected through nationwide registers. Total and cause-specific mortalities were calculated as a function of cumulative antipsychotic exposure from January 2006 to December 2010.RESULTS: Compared with age- and gender-matched controls from the general population [N = 214920], the highest overall mortality was observed among patients with no antipsychotic exposure [hazard ratio [HR] = 6.3, 95% CI: 5.5-7.3], ie, 0.0 defined daily dose [DDD]/day, followed by high exposure [>1.5 DDD/day] group [HR = 5.7, 5.2-6.2], low exposure [<0.5 DDD/day] group [HR = 4.1, 3.6-4.6], and moderate exposure [0.5-1.5 DDD/day] group [HR = 4.0, 3.7-4.4]. High exposure [HR = 8.5, 7.3-9.8] and no exposure [HR = 7.6, 5.8-9.9] were associated with higher cardiovascular mortality than either low exposure [HR = 4.7, 3.7-6.0] or moderate exposure [HR = 5.6, 4.8-6.6]. The highest excess overall mortality was observed among first-episode patients with no antipsychotic use [HR = 9.9, 5.9-16.6].CONCLUSIONS: Among patients with schizophrenia, the cumulative antipsychotic exposure displays a U-shaped curve for overall mortality, revealing the highest risk of death among those patients with no antipsychotic use. These results indicate that both excess overall and cardiovascular mortality in schizophrenia is attributable to other factors than antipsychotic treatment when used in adequate dosages.
While the implications of this study may seem to conflict with other oft·quoted papers [eg Wunderink, Harrow, etc] the outcome parameters are different – functional improvement there vs overall mortality here. Further, all of these reports can’t possibly factor in the most confounding variable of all – the unique clinical field that the patient, the clinician, and the family face at any given moment in time. So reading the blogs, the individual case reports, and the various commentaries on this topic can be confusing as they often discuss these decisons as if they fall into the domain of morality – using maintenance medication is good as opposed to using maintenance medication is bad.
“this topic can be confusing as they often discuss these decisons as if they fall into the domain of morality – using maintenance medication is good as opposed to using maintenance medication is bad.”
I think the morality issue is more about the choice to use medication, not whether someone is using it or not.
….I should have written ‘informed choice” (this is not to imply that there are no psychiatrist that give ‘informed choice’ – just that there are many situations where the severely ill and their families are not provided with ‘informed choice’.
There is an overlapping area of agreement: to try, when using these drugs, to be cautious particularly with regard to dose.
The question of whether it is OK to wait before starting drugs to see if a person can improve without them is, in my opinion, at least more open to debate than the standard treatment guidelines suggest, but this issue is often made moot by people who refuse so being able to offer options to drugs seems sound.
But when there is no objection to taking drugs, starting at a low dose and increasing slowly seems to be supported by all of the data you cite above.
If a person improves on drugs, the decision about how long to continue is fraught and I agree is best made made within the “unique clinical field” of one person’s particular experience with psychosis but, as Sally says above, at least we can allow this person and his family to participate in an open discussion about what we do and do not know.
What is surprising to me is that sometimes a person who merely suggests that we begin to factor in the findings of Harrow and Wunderink into our decision making process can be cast as having taken a moral stance against the drugs.
Sally,
I presumed it was clear that my last paragraph was advice to all concerned – “the unique clinical field that the patient, the clinician, and the family face at any given moment in time.”
Sandra,
“…sometimes a person who merely suggests that we begin to factor in the findings of Harrow and Wunderink into our decision making process can be cast as having taken a moral stance against the drugs.” Does that refer to something you heard in what I said? or is it a general observation?
Mickey,
I agree that morality seems to come into this and I have been in the odd position of being cast as both too “pro” and “anti” drug. I guess it all depends on perspective. But as someone who writes blogs and comments here on this topic, I did wonder if mine were among the blogs you had in mind that cast this in a moral light. But it is also based on general observation and personal experience.
I was just looking at the article and the other articles in that issue. I think one can look at that issue and come to the conclusion that there is no fundamental shift that has occurred in thinking about the treatment of psychosis. When you mention “oft quoted papers” by Wunderink and Harrow, I think perhaps they are oft quoted primarily in our own somewhat small little world. So when some of us shout a bit maybe it is just to be heard.
I also wish they had looked at substance use. It seems that they have the ability to do that.
Confucius say, be dismissive of and minimize hard outcomes at your (patient’s) peril. Death is a hard outcome.
Sandra,
I think you might be right that I may have a skewed window in that I keep up with the MIA site and those studies are often in emails I get at that private email address – as if I don’t know about them. I don’t so much associate them with you, but I do think of you when Open Dialogue comes up. I also know what you mean about ‘being cast as both too “pro” and “anti” drug’ because the same thing happens to me.
I’m afraid that we live in a world of Straw Men and Stereotypes these days. I do my best not to let that get under my skin, but it’s hard to do sometimes…
Alas, we do live in a world of Straw Men and Stereotypes, and characterizations of Emil Kraepelin and Eugene Bleuler are prime examples. Just to offer a minor correction to Mickey’s opening statement:
Both Kraepelin and Bleuler created natural disease concepts for dementia praecox and schizophrenia. From 1911 onward Bleuler’s schizophrenia was immediately reframed by American psychiatrists as a functional syndrome (a set of reactions or psychosexual mental mechanisms). But just like Kraepelin, Bleuler was proposing a fundamental disease concept. Both men viewed the pathophysiology as systemic (“whole body”) in some way, eventually affecting the brain but also the peripheral nervous system and other glands and organs in the body.
In December 1929 Eugen Bleuler came to the United States to visit his son Manfred, who was in training under Harvey Cushing to become a surgeon. This was not to be, for after spending 18 months in the US Manfred suffered severe injuries in a mountain climbing accident and was left with a persistent tremor that made him unfit to be a surgeon. so he eventually followed in his father’s footsteps and became a psychiatrist and noted schizophrenia researcher.
When Eugen Bleuler saw how Americans were using his term “schizophrenia” — as a syndrome with a functional basis — he was horrified. On 7 December 1929 he gave a talk to the Massachusetts Psychiatric Society and reminded the americans that “psychic causes” or “psychic mechanisms . . . do not explain the whole disease.” And then, just to bring home his point, he said: “On the whole, schizophrenia seems to be a physical disease with a lingering course, which, however, can exacerbate irregularly from some reason unknown to us, into sudden episodes, then get better again.”
Sorry to be a “smarty-pants” and jump into this far more serious discussion concerning treatment with antipsychotic medication, but these Straw Men and Stereotypes continue to be passed along in print, and it frustrates historians to no end.
For a quick check of my reference it is discussed on pp. 269-270 of my 2011 book on dementia praecox, but Bleuler’s talk was published here:
Eugen Bleuler, “The Physiogenic and Psychogenic in Schizophrenia,” American Journal of Psychiatry 10 (1930): 203-211.
..”be as informed as possible” is good advice, but a hard act to follow, given the systematic misinformation by third party industrial and academic interests, averse to giving up riches gotten from cooperating, not fighting, the present corruption in medicine and psychiatry. I try to be as informed as possible, reading also every entry on your blog, living with, accepting that life is fraught with ambiguity and that medical treatments are risky and may harm and kill the patient. The ethics in this must be to be as honest as possible about what is known, what is uncertain and what are the
… options, the possibilities, the risks. It seems to me that you, dr Nardo, and many commenters demonstrate this commendably. But there is a web holding us back from thinking freely about these complicated matters, the web woven by the language of multiple editons of DSM and ICD. I have asked some prominent psychiatrists what is schizophrenia, what etiology is known, getting blank stares, no answers. The descriptions I know already. We need to get deeper into this, not be stopped by the combined forces of money, power and inherited, dangerous words that spell doom for those so diagnosed and labelled. A cowardly psychiatry bending to power and tradition instead of searching for greater truth, is totally unscientific, not worth having.
Have you read this critique of that study?
http://www.madinamerica.com/2015/05/neuroleptic-drugs-and-mortality/
Scroll down to the section “Mortality Rates and Neuroleptic Use” for the meat of it. In summary, I find these points pretty convincing: 1)
all study participantsmost patients received some treatment, and thus it is likely that those who did not use neuroleptics refused them. This is likely to be a far different group than those who willingly took them. 2) the zero-use group was not truly zero-use. The authors did not have records of neuroleptic use while hospitalized, and thus the zero-use group may have taken them in the hospital but failed to do so after leaving the hospital (possibly resulting in severe withdrawal and serious adverse events). Indeed, point #3 says that those patients in the zero-use group who also had zero treatment at all had a much lower risk ratio (1.06). 4) Mortality due to neuroleptic exposure is likely to rise with age, yet this study cut off at 65 years old. 6) Perhaps the worst problem with the study – exposure as measured in the study was not lifetime exposure as you would assume, but exposure over the five years of the study follow up. It is entirely possible that a large number of the “no-use” group were lifetime patients who stopped neuroleptics for some reason – perhaps tardive dyskinesia – and then did not do well. To my eye, this potential makes the results for the “no-use” group totally worthless. There is no way around it with the data we are given.Thanks Ryan,
I had not seen this post. I’ll take a look…
Mickey
Richard,
I thought of you when I was writing this. Jump in whenever you want. “Smarty-Pants” AKA “expertise” always welcome.
The post on MIA that has been mentioned is generally off the mark. Many of the points made there are pedantic quibbles or tendentious speculations, rather than substantive critiques of the Swedish study. In particular, the MIA author is off the mark in his hectoring complaints that “the “cumulative antipsychotic exposure” applied only to the five-year follow-up period…” The study authors were very clear about the term cumulative antipsychotic exposure for the main group. The MIA author is throwing chaff into the discussion by substituting his own preferred meaning of the term cumulative as coterminous with lifetime exposure.
The MIA author also is dead wrong when he objects repeatedly that neuroleptic use prior to the five-year follow-up period was not considered. I will grant that there is some lack of clarity about that for the first cohort – though not nearly as much as the MIA author wants to imply. We should note, however, that the study authors were crystal clear that they considered previous antipsychotic drug use for the first-episode cases – where, by the way, the most striking mortality findings were seen. Here is their detailed description: “In order to avoid survival bias, we also conducted an analysis in a cohort of first-episode patients (N = 1230). This open cohort included all individuals in Sweden, who were between 18 and 59 years of age when entering the cohort, and who had their first-episode psychosis between 2006 and 2009 and who had also received a diagnosis of schizophrenia (in hospital treatment or outpatient treatment) by the end 2010. For these analyses, follow-up began at the date of discharge from a hospital or at the date of first psychosis diagnosis or, if the person was admitted to hospital within the first month after the first psychosis diagnosis, at the date of discharge from the hospital. In total, 2852 individuals were identified for the first-episode cohort. The individuals with disability pension due to psychosis before the beginning of follow-up were excluded (n = 501; those patients had not been in treatment contact between 1988 and 2005). We also excluded individuals who had antipsychotic medication use within 6 months before the first episode (n = 1121). After exclusion criteria, the total sample consisted of 1230 individuals.”
As I mentioned, the data for the first-episode cohort are hard to argue against, even for someone as contrarian as the MIA author. It helps to be able to see the forest for the trees. Clinicians who ignore these mortality data in schizophrenia do so at their patients’ peril. To repeat, death is a hard outcome.
When I started my graduate program in business an instructor looked out and stated: We were the best and brightest, it was sad it would be 25 years before we would be able to put to use what we were learning.
The point was we as a society are always looking to the past and what is comfortable. Changes take generations, as noted by the time lines listed by commentators.
We are the best and brightest, maybe not so young, but we seek change in a system we see as not reflecting the very best for patients and society. The drive to push back against an all or nothing, this or that system, will take time.
I can only hope this blog, and others like it, will produce the balanced approach in treatment we all seem to agree would be best for not only patients, but society.
Steve Lucas
Does anyone know what the hypothesis is as to why they think antipsychotic medication would prevent cardio-vascular death ?
Wait, so 39% of first-episode schizophrenia patients in this cohort had antipsychotic use prior to their first episode? Is that normal? And 39% appears to be low because it only counted those with antipsychotic exposure in the 6 months prior to the study. Use before that was not considered. So, after we exclude those patients and others, we are down to a mere 1230. All it would take to totally skew the results with that number are very small differences in the composition of the cohorts. And indeed, there are very large differences from what little information we are given. In this way, the first-episode cohort magnifies the other problems of the study. It also compresses the time frame, further reducing any negative effects of neuroleptics. For example, under the criteria given, a patient may have only had one year of neuroleptic treatment in the 5 year period.
On the subject of differences in the groups, first, standard treatment is to use neuroleptics. Thus, it can be assumed that patients who did not use them either 1) did use them in the hospital but did not after discharge (resulting in withdrawal), 2) did not use them for medical reasons (perhaps cardiovascular reasons, etc), 3) refused them. We already know that patients who refuse all treatment do not do well. Their refusal to accept treatment may be a reflection of the severity of their condition. As Sally noted, and is shown at the MIA link above, the “no use” group is rather strange in regards to cardiovascular death. The study would seem to indicate that low use of antipsychotics lowers cardiovascular death. But that is the opposite of what we know. The alternative explanation is that the “no use” group had more cardiovascular problems, and those could be a part of why they did not use antipsychotics (or it could reflect their past use). And this applies to all causes of mortality, except perhaps suicide. Those with already existing medical problems are more likely to not be given drugs, causing the “no drug” group to consist of much less healthy people. The increase in suicides would easily be explained by patients undergoing withdrawal after leaving the hospital and/or patients who are the most likely to reject treatment are also the most likely to commit suicide.
To Ryan. So called at risk youngsters in Early Intervention studies spread from Australia and USA to Europe and beyond are treated with neuroleptica, trying to prevent transition to psychosis. Some psychiatrists are able to be enthusiastic about their research without even mentioning the use of dangerous drugs in “prepsychotic” people and the disappointingly weak results. As for excess mortality in patients diagnosed with schizophrenia highest early on, loss of hope may be a major factor in high rates of suicide.
http://iepa-vcl.eppic.org.au/content/excess-mortality-causes-death-and-life-expectancy-main-groups-patients-recent-onset-mental
http://forskning.ku.dk/find-en-forsker/?pure=da%2Fpublications%2Fexcess-early-mortality-in-schizophrenia(ef27e3ce-4ac9-42be-90ed-0c79537d8a29).html
Sorry for the faulty link, and hoping that this will work:
http://www.annualreviews.org/doi/abs/10.1146/annurev-clinpsy-032813-153657
Thanks for those links. The first one hinted at something I intended to say but forgot: the possibility that those refusing treatment are more likely to be illegal drug users. I do not know if there is any data on this in this study or elsewhere, but it seems likely to me that users of illegal drugs like amphetamines would be more likely to refuse/cooperate with treatment and go back to using. Controlling for this might be difficult because they may not even share their past/current drug use.
I think this and other reasons I mentioned before just illustrate the difficulty of extrapolating meaningful results for “no use” as a single group by doing observational studies like this one. And this is not to say that I believe one way or the other with any certainty. I just think this data is particularly faulty. Antipsychotics may be efficacious for short term use, and they may actually prevent suicides, but I find this data unable to provide any reliable evidence for that. I am very much in line with the Moncrieff mindset that antipsychotics have a simple drug effect and may be useful in that capacity, but should never be thought of as a targeted treatment. They are just incapacitators.
The Moncrieff idea is bosh. She holds that no drugs have specificity of action for particular psychiatric conditions. Instead, everything is supposed to happen through some never-specified, vague, psychotropic action that somehow alters the patient’s mental state in a way that the patient interprets as helpful. Two examples unravel that specious proposition. First, we stumbled upon the antidepressant property of imipramine in the 1950s precisely because it didn’t give benefit to patients with schizophrenia whereas it was seen to have remarkable antidepressant properties. A more current example is the differential effectiveness of the benzodiazepine drug lorazepam for catatonia, whereas antipsychotic drugs may well make the situation worse.
All,
When I posted this, I didn’t go through the machinations, but I thought about population studies in general. I’m embroiled up to my eyeballs in another one right now. In some ways, it’s easy to have the fantasy that if we just had complete records, we could find the answers we need. That’s one of the reasons we’ve traditionally looked to Scandanavia for this kind of data. Scandanavians “stay put,” and have centralized and comprehensive record keeping systems that have been around for a while. But retrospective population studies are neither clinical trials nor case studies. We’re relying on parameters set in the past, and they rarely match what we’re looking for, so they are often frustrating. I posted this one because I thought they had given it a fair shot, and I couldn’t locate any warning signs of large scale biasing. But population studies are often like a Rorschach ink blot, offering lots of opportunity for speculation that can’t possibly be chased down. I don’t know if the “U” shape would hold up were we to do the perfect version of this study, but I felt that it was a value added study in what it didn’t confirm – “The more antipsychotic you take, the worse you get.” I’ve heard that throughout my career, and acted on it by aiming for minimal to no maintenance medications.
But, to be honest, I really haven’t seen the predicted devastation and deterioration. So I found this population study interesting. But I still believe that these patients are a heterogeneous group, and they are best served by being followed long term by a consistent clinician no matter what their treatment choices at any given point along the road. Over time, the best choices are made by a therapist/patient dyad who’ve known each other over time and have followed the course of the psychotic illness together. In that circumstance, population studies and Clinical Trials become more like a reference library than “treatment guidelines.” That’s how we follow people with Diabetes, Heart Disease, Chronic Arthritis, etc. There may be a number of guidelines around, but the individual case studies bespeak the real meaning of “personalized medicine”…
Amen to what Dr. Mickey has just stated. By the nature of clinical research, all studies will be imperfect in the eyes of professional cavilers like the MIA author. Trouble is, professional caviling doesn’t move the ball down the field. No clinical study will succeed in directly controlling for all potential confounds or in capturing all conceivably relevant sources of data. That is just as true, by the way, of the Wunderink studies as it is of the Tiihonen studies. In each of those examples, our task is to see the forest for the trees, to internalize their principal messages when advising our patients but without necessarily endorsing all their flourishes, and to inform the design of the next round of studies with insights obtained from the earlier ones. If we do that, we remain true to the pragmatic nature of clinical science and we make the scolding cavilers irrelevant.
I am not sure that you understand Moncrieff’s argument, Dr. Carroll. Or at least my interpretation of what I read. Her argument is not that there is one generic, vague psychotropic action that is the same for every drug, but rather that each drug class has specific effects that may or may not be helpful for particular conditions, but none of them are specific to the conditions, rather just vague modifiers of brain behavior that may be perceived as helpful by some patients and for some conditions. Thus, benzodiazepines certainly relieve anxiety, but they do not do so by actually fixing the anxiety, but rather removing the brain’s ability to experience it. Antipsychotics do not specifically treat schizophrenia, but may be useful in calming symptoms, particularly for violence, by making the patient incapable of expressing violence.
Imipramine is an interesting case because its efficacy for depression is very tenuous. The old antidepressants suffer from the same methodological flaws as the SSRIs (or worse). It is notable that the original case reports of the wonders of imipramine sound much more like mania than actual recovery, something imipramine is well known for inducing. Moncrieff would never argue that lorazepam and antipsychotics work in the same way, only that neither has effects that treat specific conditions – rather they only treat specific symptoms. Of course they work differently – they are two entirely different drug classes with totally different mechanisms. I would be the first to admit that benzodiazepines are in fact very effective for their intended condition. But there is nothing specific about them – they just provide symptom relief. Increase the dose and you get euphoria. This is why they are commonly abused recreationally. That said, the main effect of SSRIs seems to be a “numbing” of emotions, and that could be at play with imipramine as well. This numbing could be seen as advantageous to some who are depressed. They now no longer care about their depression. Although to others this numbing may itself be depressing.
As an aside, where you (and many researchers) state that antipsychotics “may make the situation worse” with catatonia, I can assure you with first hand experience that they do without a doubt. I witnessed a fairly moderate dose of an antipsychotic induce severe catatonic stupor that was alleviated by lorazepam (although that created a manic state). It was a scary thing to see. And it was consistent. There was nothing magical about the effect – the person was likely just experiencing a higher dose than expected for biological reasons. Antipsychotics are just brain disablers on a sliding scale…increase the dose enough and you will make someone a vegetable. The research evidence for this is enormous, and I find it hard to believe that some professionals are so in denial about this.
In response to Mickey: the patient/therapist dyad is a sensible place to put your trust, and from my experience I believe your heart and your instincts are in the right place. However, the patient and therapist make choices informed largely by the data we have from studies like this one. If the data is not trustworthy or wrong, then they make poor choices. If a patient and therapist make the wrong choice, there is often no way for them to actually know this.
On your general note about population studies: observational studies like this one are very seductive because they seem like treasure chests of data. But they should be approached with great caution. There are reasons why we do randomized trials, and observational studies toss those reasons aside. The resulting data may be meaningful, but it also may be a minefield.
This last comment is a good example of professional caviling. The comment contains so many tendentious tropes (over-used plot devices) that I won’t try to counter them all. Just to illustrate, I will say that on the subject of imipramine the writer could not be more wrong. Psychiatrists who treated depression before 1987 will recognize the errors. When the writer says a typical response to imipramine looks like the induction of mania, we can be certain that a fevered imagination is working overtime, fueled by a tedious bias. This kind of massive distortion traces back to the perverse Moncrieff narrative. I rest my case about the irrelevance of such misinformed statements – made, by the way, with breathtaking assuredness by someone who won’t properly identify himself or the professional experience that he thinks allows him to spread such misinformation. An aphorism comes to mind: a little knowledge is a dangerous thing.
“Imipramine is an interesting case because its efficacy for depression is very tenuous. The old antidepressants suffer from the same methodological flaws as the SSRIs (or worse).”
This isn’t true either. Most of the TCA studies weren’t weakened by a definition of depression that became increasingly meaningless.
Dr. Carroll was correct about pre-1987 psychiatry. When we gave TCAs the general concern was efficacy and anticholinergic side effects not mania. BTW the FDA blew it with Merital.
I do not want this discussion to get heated as some others on this site do, so I will keep my tone very civil, as I think Mickey’s blog is a great place for discussion. Nowhere did I say that the typical response to imipramine is a manic reaction. That would be false. Instead, I said the original case reports of its wonderful powers were typical manic reactions. The drug I was thinking of was actualy Iproniazid, so I will offer that correction. Instead, the original reports on Imipramine were merely implausible (patients depressed for years were totally cured in 2-3 days!) But this is long ago history. It is important, but largely a footnote.
I do take exception to the mischaracterization of my position. It is not helpful to take my statement, blow it up into a straw man argument, and then claim I have no credibility based on that straw man, finishing with a rhetorical flourish of “a little knowledge is a dangerous thing”. Of course manic states are not the typical response to any antidepressant. But they do occur and they are much more prevalent than the clinical trials report, especially with SSRIs (you can disagree with that and I do not mind). Most people do not experience manic states, but a small portion do. Just as a small portion feel very little. Of course, TCAs are not SSRIs, and the rates of mania appear to be much lower, but they are still there.
In response to James: I actually agree that the definition of depression has been weakened and this is unhelpful. But when I speak of methodological weaknesses, I speak of the almost total loss of placebo blinding in all trials. I speak of the very low efficacy against placebos. The old antidepressants and SSRIs are considered largely the same in effect in the research literature, often differing only in the more dangerous side effects of the old drugs. Even if you believe that SSRIs do have some treatment effect, the best data says that effect is low. The NNT is 10. Something like 6 of 10 people will get better on SSRIs. Do doctors really think they can pick out the 1 out of that 6 that is having a true drug effect? Doctors want to believe that the drugs they are giving are helping – after all no one really wants to be dishing out placebo pills with side effects. But is it not also a positive sign that 85% or more of the treatment effect is a result of the patient’s interactions with you and/or their expectation that they will get better largely based on that interaction?
I am digressing, but mainly I just want to reiterate that the characterization of my position was not fair. If you read the actual history on Imipramine, the original reports were implausible, so perhaps it was all made up. The descriptions of patient’s recoveries were implausible. One patient was described as being “cured” of homosexuality. Finally, I am aware there are meta-analyses of Imipramine that say there is no risk of mania, but I do not believe those because the studies they analyze are notorious for under-reporting of side effects like mania. There are plenty of case studies showing Imipramine’s mania inducing effects, particularly during withdrawal. Though I will not press on about Imipramine and mania because the anecdote I remembered was about a different drug and Imipramine’s ability to induce mania is low compared to other antidepressants.
Thanks,
I think we’ve got enough info at this point to think about…