Well, I didn’t plan it, but this post actually turns out to be a sequel [maybe prequel] to another post from two weeks ago [probably approximates zero…]. There, I was reporting on an analysis that showed that the major use of antipsychotics in children is not to treat psychosis, but rather to control disruptive impulsiveness in developmentally challenged children – aka behavior control. Remember that the first Atypical Antipsychotic, Risperdal®, came out of the gate looking for an FDA Approval for that very indication with a study by Janssen’s Risperidone Disruptive Behavior Study Group [Double-Blind, Placebo-Controlled Study of Risperidone for the Treatment of Disruptive Behaviors in Children With Subaverage Intelligence 2002] and were turned down. That study was later repurposed, ghost written, and republished under Dr. Joseph Biederman’s name to support a claim of efficacy in his proposed Bipolar Child diagnosis [Risperidone for the treatment of affective symptoms in children with disruptive behavior disorder: a post hoc analysis of data from a 6-week, multicenter, randomized, double-blind, parallel-arm study 2006]. Many of us believe that the Bipolar Child designation was more a rationalization to use Antipsychotics in children for behavior control than a valid psychiatric condition.
Toronto StarBy David Bruser and Jesse McLeanJuly 31, 2015A medical journal article co-authored by SickKids hospital’s top pediatrician was manipulated by a drug company and understated the risks of a powerful antipsychotic used to treat kids with behavioural problems, the former head of the U.S. drug regulator alleges. The 2003 article concluded there was no correlation between long-term use of Risperdal and an increased risk of certain side-effects, including the growth of breasts in boys. The listed authors included respected experts in the pediatric field — Toronto’s Dr. Denis Daneman and two U.S. doctors — and three employees from Janssen, which makes Risperdal.
The medical article was singled out in recent U.S. lawsuits against Janssen as an alleged example of drug company influence on doctors and published research. A court exhibit shows a draft of the study describing an association with certain side-effects — a finding that a Janssen employee internally flagged as “significant.” This information is not in the published version of the article.
“My name is on an article in which there is some data that has been left out. That, to me, crosses a line,” Daneman, pediatrician-in-chief at the Hospital for Sick Children, told the Star…
This is a story about the role of a drug company in the production of a scientific journal article. The alleged manipulation of the medical article is outlined in a report by expert witness Dr. David Kessler, former head of the U.S. Food and Drug Administration and a pediatrician by training. The report, filed in U.S. court, said Janssen “controlled and influenced” more than 40 manuscripts, including the one co-authored by Daneman. Kessler called the company’s alleged promotion of the drug for unapproved use in vulnerable children “deeply troubling”…
An independent biostatistician is now reanalyzing the 2003 study’s data to determine whether the original results stand or if they need to be corrected on the public record, Daneman said. “If, indeed, these allegations are true, then I would feel used,” he said, adding that his contributions to the paper “were made in good faith and based on the assumption that my colleagues and I had access to all the relevant data.” He said he was not included in internal Janssen discussions about certain revisions to the article. “There is this deception there that is intolerable,” he said…
In a 2012 deposition where he was questioned by a U.S. plaintiff’s lawyer, Daneman, a pediatric endocrinologist, agreed the study he participated in had data calculation errors that had the effect of understating the frequency of side-effects. In an interview with the Star, Daneman said there was no intent on his part to exclude significant results…
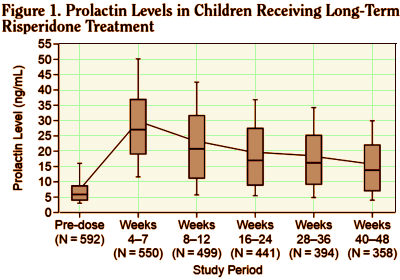
The 2003 study analyzed results from five Janssen clinical trials to investigate prolactin levels in children and teens taking Risperdal, and “explore any relationship with side-effects hypothetically attributable to prolactin.” Daneman said his role in the study was commenting on parts of the article and suggesting changes and that somebody else wrote it. He told the Star he was paid about $1,000 by Janssen for his participation. “I gave the money to charity because of the concerns that had been raised about potential conflicts of interest,” he said…
by Findling, Kusumakar, Daneman, Moshang, De Smedt, and Binder.Journal of Clinical Psychiatry. 2003 64[11]:1362-1369.
BACKGROUND: This analysis was designed to investigate prolactin levels in children and adolescents on long-term risperidone treatment and explore any relationship with side effects hypothetically attributable to prolactin [SHAP].METHOD: Data from 5 clinical trials [total N = 700] were pooled for this post hoc analysis. Children and adolescents aged 5 to 15 years with subaverage intelligence quotients and conduct or other disruptive behavior disorders received risperidone treatment [0.02-0.06 mg/kg/day] for up to 55 weeks. Outcome measures analyzed included serum prolactin levels, reported adverse events, and the conduct problem subscore of the Nisonger Child Behavior Rating Form.RESULTS: Mean prolactin levels rose from 7.8 ng/mL at baseline to a peak of 29.4 ng/mL at weeks 4 to 7 of active treatment, then progressively decreased to 16.1 ng/mL at weeks 40 to 48 [N = 358] and 13.0 ng/mL at weeks 52 to 55 [N = 42]. There was no relationship between pro-lactin levels and age. Females returned to a mean value within the normal range [</= 30 ng/mL] by weeks 8 to 12, and males were close to normal values [</= 18 ng/mL] by weeks 16 to 24. At least 1 SHAP was reported by 13 [2.2%] of 592 children. There was no direct correlation between prolactin elevation and SHAP.CONCLUSION: With long-term risperidone treatment in children and adolescents, serum prolactin levels tended to rise and peak within the first 1 to 2 months and then steadily decline to values within or very close to the normal range by 3 to 5 months.
My point in this post isn’t just about Dr. Daneman’s culpability in this obviously wrongly interpreted study twelve years ago. He was a key link in Janssen’s campaign to maximize its Risperdal® profits by developing an off-label pediatric market. I admit that this is a particular sticking point for me. I sat through the TMAP trial in 2012 and listened to drug reps and marketing managers describe campaigns targeting child psychiatrists and pediatricians to use Risperdal® off label – campaigns based on outright lies [see on every call…]. But my main point is Dr. Daneman’s argument that Janssen with-held evidence from him. It wasn’t his fault, he didn’t write that paper. He just commented on it. He even gave away the money he received.
Daneman said his role in the study was commenting on parts of the article and suggesting changes and that somebody else wrote it.
Sorry, the comment form is closed at this time.