While it may seem that with all the attention being focused on Data Transparency for Clinical Trials and things like the AMA voting to oppose Direct-to-Consumer advertising, we are iterating towards a time when the battle against the corruption of medical practice by commercial interests is making some real progress. 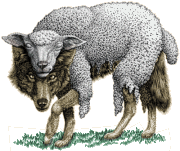 But it seems to me like there’s always the rumbling of distant thunder. As physicians, we should be attuned to such early warnings. After all, there’s an important medical principle involved – Preventive Medicine. While talking about such things can begin to sound like early psychosis, the alternatives are too well known to ignore. Particularly dangerous are things that look innocent, maybe even like progressive reform to solve some contemporary problem, but later turn out to have been the proverbial wolf in sheep’s clothing.
But it seems to me like there’s always the rumbling of distant thunder. As physicians, we should be attuned to such early warnings. After all, there’s an important medical principle involved – Preventive Medicine. While talking about such things can begin to sound like early psychosis, the alternatives are too well known to ignore. Particularly dangerous are things that look innocent, maybe even like progressive reform to solve some contemporary problem, but later turn out to have been the proverbial wolf in sheep’s clothing.
In psychiatry, we have a number of examples, but the one that jumps off the page is the Texas Medical Algorithm Project [TMAP]. When the Atypical Antipsychotics were first introduced, we couldn’t have been more primed and ready. They were effective alternatives to our older drugs but with a markely reduced incidence of neurologic side effects. The experts agreed and said so in widely publicized guidelines. Since the majority of antipsychotics are prescribed in public systems, it only made sense to insure that the less fortunate treated in these public systems have access to this medical breakthrough – implemented in Texas through the TMAP Guidelines which prescribed ‘try the Atypical Antipsychotics first.’  It was such a good idea that the Texas Mental Health officials became evangelical, ultimately spreading the gospel to about a third of our states [with a bit of financial help from the manufacturers]. When it was all said and done, the Texas Medicaid program was all but bankrupt; it became clear that the Atypicals were neither more effective nor better tolerated than the older drugs; and they had some toxic side effects all their own.
It was such a good idea that the Texas Mental Health officials became evangelical, ultimately spreading the gospel to about a third of our states [with a bit of financial help from the manufacturers]. When it was all said and done, the Texas Medicaid program was all but bankrupt; it became clear that the Atypicals were neither more effective nor better tolerated than the older drugs; and they had some toxic side effects all their own.
And now? Well there’s the Trans-Pacific Partnership that many worry will bring many rewards to the pharmaceutical industry including even longer periods of patent exclusivity. I’ll have to admit that my understanding of this trade agreement remains embryonal even after several attempts to understand it. Some of the problem is their secrecy, but I fully admit absolutely no expertise in the area. But there’s another one that, though my understanding of the basics is equally poor, I think the rat I smell is very real. It’s a bill in Congress called the 21st Century Cures Act that has passed the House already [see The F.D.A.’s Medical Device Problem, 21st Century Cures Act: A huge step backward for FDA standards, and Will the 21st Century Cures Bill Lower Standards for Some Drug Approvals?]. The sheep’s clothing is that getting drugs approved is a long, slow process. And patients, particularly patients with desperate or terminal illnesses understandably don’t want to wait. But the very large wolf is that the proposed solution is "fast-tracking" drugs and devices, without the preapproval Clinical Trials, relying on antedotes and other indirect evidence. That opens Pandora’s Box allowing dangerous drugs on the market, particularly the kind of drugs used to treat dire illnesses.
An FYI:
http://mobile.nytimes.com/2015/11/26/opinion/treating-mental-illness-in-new-york-from-all-angles.html?emc=edit_th_20151126&nl=todaysheadlines&nlid=43412594&_r=1&referer
https://thrivenyc.cityofnewyork.us
Re ThriveNYC – a quick glance at the roadmap reveals expected concerns: “Starting immediately, the City will hire 100 School Mental Health Consultants (SMHCs) who will work with every school citywide to ensure that staff and administrators have an outlet to connect students with the highest immediate needs to care.”
“Closing Gaps on Maternal Depression: New York City is setting a goal to screen and treat all pregnant women and new mothers for pregnancy related depression.”
I’m sure there’s many more – wolves in sheeps clothing…