Don’t be alarmed. This isn’t one of those long boring posts with formulas and numbers. The graphic below is just window dressing to make one simple point:
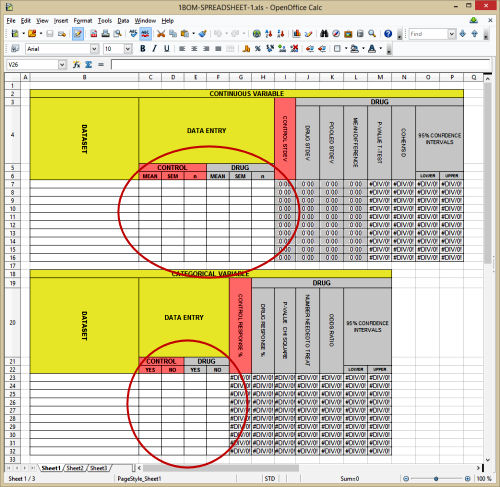
I’ve been writing a mini-tutorial for evaluating Clinical Trials, and this spreadsheet will generate the needed information in the absence of Data Transparency. All we need for the Continuous Variables is the MEAN, the Standard Deviation or the Standard Error of the Mean, and the Number of Subjects for the control [placebo] and the drug groups. And for the Categorical Variables, we need even less – just the tally of yeses and nos for both. Those are not esoteric numbers – a basic minimum of information.
In defending their claim that the raw data from these Clinical Trials is proprietary and can be kept from scrutiny, the pharmaceutical industry makes two arguments: protecting their subject’s privacy and withholding commercially confidential information [CCI in slang]. But neither argument pertains to the simple information to plug into that spreadsheet. And yet one is surprised with how often that data isn’t available in an article. The graphic is meant to demonstrate how minimal the request – three numbers for each group in the Continuous Variables and only two numbers for the Categorical Variables in each group.
Sorry, the comment form is closed at this time.