PSYCHIATRICNEWSby Aaron LevinJune 16, 2016… As for treatment, only two drugs are approved for use in youth by the Food and Drug Administration [FDA]: fluoxetine for ages 8 to 17 and escitalopram for ages 12 to 17, said Wagner. “The youngest age in the clinical trials determines the lower end of the approved age range. So what do you do if an 11-year-old doesn’t respond to fluoxetine?”
One looks at other trials, she said, even if the FDA has not approved the drugs for pediatric use. For instance, one clinical trial found positive results for citalopram in ages 7 to 17, while two pooled trials of sertraline did so for ages 6 to 17. Another issue with pediatric clinical trials is that 61 percent of youth respond to the drugs, but 50 percent respond to placebo, compared with 30 percent among adults, making it hard to separate effects.
When parents express anxiety about using SSRIs and ask for psychotherapy, Wagner explains that cognitive-behavioral therapy [CBT] takes time to work and that a faster response can be obtained by combining an antidepressant with CBT. CBT can teach social skills and problem-solving techniques as well. Wagner counsels patience once an SSRI is prescribed.
A 36-week trial of a drug is too brief, she said. “The clock starts when the child is well, usually around six months. Go for one year and then taper off to observe the effect.” Wagner suggested using an algorithm to plot treatment, beginning with an SSRI, then trying an alternative SSRI if that doesn’t work, then switching to a different class of antidepressants, and finally trying newer drugs.
“We need to become much more systematic in treating depression,” she concluded.
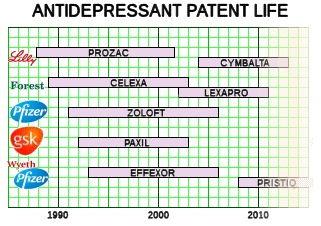
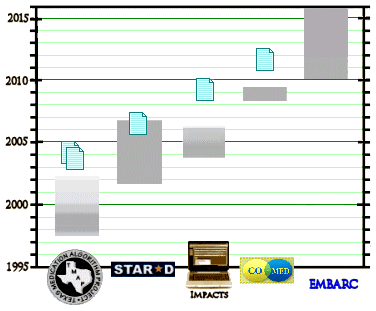
Wagner’s 2016 APA presentation sounded like a direct transcript from those days – in spite of the fact that none of the studies in between really bolstered the idea that this path lead much of anywhere. STAR*D claimed to support sequencing, but it had methodologic problems, a huge dropout rate, and was never fully reported [see a thirty-five million dollar misunderstanding…]. She mentioned none of this and, at least in the PSYCHIATRICNEWS report, didn’t present her argument with evidence that her recommendations got results.
She referred to the FDA Approved drugs ["fluoxetine for ages 8 to 17 and escitalopram for ages 12 to 17"] and recommended not feeling limited to just these two – pointing out several clinical trials that were positive ["one clinical trial found positive results for citalopram in ages 7 to 17, while two pooled trials of sertraline did so for ages 6 to 17"]. She’s referring to studies in 2003 and 2004 where she is the first author, one being recently heavily criticized [see The citalopram CIT-MD-18 pediatric depression trial: Deconstruction of medical ghostwriting, data mischaracterisation and academic malfeasance] and another also widely questioned [at least she left out Paxil Study 329]. So based on her own ghost written industry funded studies from twelve+ years ago, she is encouraging full speed ahead with the aggressive TMAP guidance.
But, to be honest, that’s not what actually got my attention. It was what she didn’t mention. The most obvious thing is the Black Box Warning of potential suicidality in children and adolescents present since 2004. Then she refers to her short term clinical trials from early days, and nothing since then! Both trials come up in the deposition, and her involement was slim to none, and she remembers nothing much about them. She discounts the FDA, evokes these two early ?? trials, and doesn’t mention anything else. There’s no, "In our clinical experience…" or "In follow-up long term studies…" or "In the Galveston clinic, we’ve seen…"
You mentioned “…she refers to her short term clinical trials from early days, and nothing since then!” Forest Laboratories would have seen no reason to run more clinical trials as long as they were satisfied with their market share, thanks to the KOL “authorities.” Speaking of market share, as I pointed out recently, the FDA has reported that between 2005 and 2010 well over 750,000 patients up to age 17 received escitalopram, including almost 160,000 under age 12. AND THAT WAS ALL OFF-LABEL, BEFORE THE DRUG WAS APPROVED, BUT ONLY FOR AGES 12 AND UP. We ain’t talking peanuts here.
Speaking of psych drugs in kids and teenagers, how’s that “neuroprotection” theory working out?
http://jop.sagepub.com/content/30/2/204