 The Placebo Effect in Clinical Trials is more than a philosophical matter. It’s a practical consideration, something that is regularly subtracted from the overall effect in presenting the results, yet it’s poorly understood itself. For some, it’s seen as the sampling phenomenon referred to as the regression to the mean [see in the land of sometimes[4]…]; for others, it’s suggestability and expectation; and there’s also a literature exploring a genetic/neurobiological component. But just looking at the graphs, one might well conclude that simply being in as trial is itself a therapy. The placebo effect is certainly a prominent effect in the adolescent antidepressant trials that have frequently graced these pages. Here’s an interesting deconstruction specifically attempting to parse out the role of expectation of benefit in these trials:
The Placebo Effect in Clinical Trials is more than a philosophical matter. It’s a practical consideration, something that is regularly subtracted from the overall effect in presenting the results, yet it’s poorly understood itself. For some, it’s seen as the sampling phenomenon referred to as the regression to the mean [see in the land of sometimes[4]…]; for others, it’s suggestability and expectation; and there’s also a literature exploring a genetic/neurobiological component. But just looking at the graphs, one might well conclude that simply being in as trial is itself a therapy. The placebo effect is certainly a prominent effect in the adolescent antidepressant trials that have frequently graced these pages. Here’s an interesting deconstruction specifically attempting to parse out the role of expectation of benefit in these trials:by Bret R. Rutherford M.D., Joel R. Sneed Ph.D., Jane M. Tandler H.S., David Rindskopf Ph.D., Bradley S. Peterson M.D. and Steven P. Roose M.D.Journal of the American Academy of Child & Adolescent Psychiatry. 2011 50[8]:782-95.
Objective: This study investigated how study type, mean patient age, and amount of contact with research staff affected response rates to medication and placebo in acute antidepressant trials for pediatric depression.Method: Data were extracted from nine open, four active comparator, and 18 placebo-controlled studies of antidepressants for children and adolescents with depressive disorders. A multilevel meta-analysis examined how study characteristics affected response rates to antidepressants and placebo.Results: The primary finding was a main effect of study type across patient age and contact amount, such that the odds of medication response were greater in open versus placebo-controlled studies [odds ratio 1.87, 95% confidence interval 1.17–2.99, p = .012] and comparator studies [odds ratio 2.01, 95% confidence interval 1.16–3.48, p = .015] but were not significantly different between comparator and placebo-controlled studies. No significant main effects of patient age or amount of contact with research staff were found for analyses of response rates to medication and placebo. Response to placebo in placebo-controlled trials did significantly increase with the amount of therapeutic contact in older patients [age by contact; odds ratio 1.08, 95% confidence interval 1.01–1.15, p = .038].Conclusions: Although patient expectancy strongly influences response rates to medication and placebo in depressed adults, it appears to be less important in the treatment of children and adolescents with depression. Attempts to limit placebo response and improve the efficiency of antidepressant trials for pediatric depression should focus on other causes of placebo response apart from expectancy.
"Medical ManagementPsychotherapy Experience in protocols in depressed adolescents suggest that patients and families expect psychotherapy and are reluctant to consider a course of medication treatment alone, especially where the medication may be solely placebo. On the other hand, a provision of treatment with a psychotherapy which, in retrospect, turned out to be extraordinarily efficacious might well preclude the demonstration of a real, significant, and clinically meaningful medication effect. There are currently several research groups beginning the process of examining different specific psychotherapies [e.g. cognitive behavioral and interpersonal] for adolescent depression. As of yet, however, there are no completed controlled studies which would suggest a "reference" psychotherapy treatment. The present study will include supportive psychotherapy, similar to the management as described by Fawcett in Appendix G.
Weekly visits will consist of a 45 minute visit with the therapist. In unusual circumstances, emergency contact of greater duration is permitted. Duration of all contact including phone calls will be systematically documented."
"3.5.4 Other Protocol-specified Therapy
Supportive psychotherapy for the depressive episode was provided in a manner similar to that described by Fawcett and coworkers in the Adolescent Depression Collaborative Research Group.[10] Psychotherapy was intended to provide the psychosocial interaction between the patient and the therapist that would permit observation of any pharmacotherapeutic effect of the study medication. Therefore, the sessions were to focus on providing supportive therapy rather than implementing interpersonal or cognitive/behavioral strategies. At each weekly visit, the patient had a 45-minute visit with the therapist. However, emergency contact of greater duration was permitted under unusual circumstances."
DO’S:
-
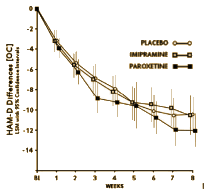
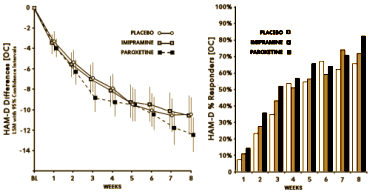
the magnitude of the change can hardly be explained simply by regression to the mean
-
it is highly unlikely that the change is due simply to expectations or suggestion
-
this cohort had been depressed for over a year on average before the study, so natural course of disease also seems an unlikely explanation
-
the supportive psychotherapy of 8 weekly sessions fits traditional parameters for therapy with depressed adolescents
So, 1boringoldman, are you suggesting that the placebo effect is in fact an artifact of the clinical trial itself? and the way the study was conducted? and that the magnitude of the effect might correlate with how the adolescents were approached? and that Study 329 was a particularly effective version? Sure that’s what I think, but I can’t go further than speculate or hypothesize because I don’t have any other protocols. And beyond that, I have no way of knowing what was actually done in the studies themselves. But a supportive, non-confrontational psychotherapeutic stance has been effective with adolescents in my own experience, so of course that’s what I think even though it’s an antecdotal opinion [don’t discount it until you read Fawcett].
The placebo effect is a result of how loosey goosey everyone in the pool DSM is. If hypertension medication studies included 125/85 then there would be a lot of placebo effect as well.
I’m kind of fed up reading major journal articles where I can immediately spot the design flaw in a major study without even picking up a calculator. Example:
http://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2016.15060800