Posted on Wednesday 29 June 2016
Home after a long bus ride on a choir trip, she was in an agitated state. Apparently one of the neighborhood girls had been telling increasingly exaggerated tales, stories our daughter knew were untrue. "She’s just a liar! Why does she do that?" And nothing we said made a dent in her consternation.The next day, we were on the front porch and my wife mentioned another neighborhood kid, an adopted girl who was headed for trouble. She’d announced to my wife that she’d met her "real" mother who was a "street walker" – this time something we knew wasn’t true. Our daughter, piped up from across the porch where she had seemed lost in a magazine and said matter-of-factly, "Oh she didn’t really meet her real mother. That’s just what she wishes was true," and returned to her magazine. But there was more to come.
On the weekend, she decided to go sailing with us – a rare treat in an age where being with friends had moved center stage. It was a good sailing day, and we’d had a fine adventure getting tangled up in a regatta of smaller boats – "gnats!". We were on a long tack back to the dock when she announced out of the blue, "All lies are wishes. I think I’m right about that." It was said as a challenge, so we joined in the game. We offered counter examples that she shot down with increasing confidence. "I’m telling you, I’m right about this."
With the acquisition of meta-thought [abstract reasoning], the adolescent finds him-or-her-self in a new world [or an old world with a new look]. They may not yet have a knowledge or experience base, but they can think as well as their parents, and seem to know it ["I’m telling you, I’m right about this."]. And they can drive parents to distraction with their endless arguing and logic games. Little wonder Anna Freud pointed to rationalization as the primo defense mechanism of adolescence. But beyond that, they now can see our flaws and foibles and gladly point them out if given half a chance, as the former authority of parenthood fades.
Erickson described the developmental task of adolescence as identity formation. The adolescent’s abstract musings quickly turn to the question of "What am I?" Blos described it as the second separation/individuation [the first being the coming into personhood at age two]. And like the two year old’s "No" as the harbinger of change, the adolescent often starts the identity process with something similar, the rebellious "I don’t yet know what I am, but I’m sure not what you think!" And there may be any number of trial identities before one fits. In my vignette about my daughter, the first girl [the "liar" on the bus] is now a successful gay married woman and I suspect her self-important exaggerations at thirteen were related to not knowing how to fit into the culture of the time. The second girl did go on to prostitution and other such, dying in her late twenties from a unintended narcotic overdose during a relapse – all in spite of her parents somewhat heroic attempts to turn things around. With the identity comes the related character or personality formation. In adolescence, all of those character traits that seemed kind of fluid in childhood begin to condense into the a predictable and fixed set of ways of doing, being, and reacting that we call the person·ality.
And finally, there’s interpersonal life ["… a rare treat in an age where being with friends had moved center stage"]. The adolescent drifts away from family life into that place of their own called adolescence, where the people that matter are peers [and the dominant culture is guaranteed to differ from the teen culture of their parents]. Even for the introvert who stays apart, peers remain the audience being played to in the mind. Adults think of it as a phase they’re going through on the way to a place in society, but for many occupants, it feels like [and may actually become] a final destination. In the finale of the classic teen musical "Grease," they sing exuberantly "We’ll always be together" – which is rarely true in fact. Yet for many, it’s etched in memory just as the song describes it. If you don’t know that, go back to your high school reunion and be awed by the floods of memories and emotions.
So what’s a quasi-psychodynamic overview of some broad issues of adolescence doing here in the middle of a blog about corruption in Randomized Control Drug Trials? I try to stick to that topic, even though I’m really speaking in my second language [mostly acquired in my latter years]. But as I’ve read all these RCTs about antidepressants in youth, I’ve had a growing frustration which came to a head with the PSYCHIATRICNEWS article about Karen Dineen Wagner’s remarks at the recent APA meeting [see a blast from the past…].
By my read, adolescence is a critical crossroad in development. It’s a period when change is in the air, a time with an opportunity to set right the unnecessary baggage carried forward from childhood. It’s also a time with its own shoals and pitfalls that may become incorporated into the adult personality structure and carried forward for a lifetime. Some change in adolescence is luck – an encounter with a teacher, a reparative friendship, a romance, and a myriad of other possibilities. But other adolescents flounder helplessly – developing symptoms, acting out, acting up, withdrawing, becoming depressed – signals for needed help.


 In 1997-98, TMAP, with pharmaceutical industry funding, began working on the Texas Children’s Medication Algorithm Project [TCMAP]. An "Expert Consensus" panel was assembled to determine which drugs would be best for the treatment of mental and emotional problems in children and adolescents. The panel consisted almost exclusively of persons already involved in TMAP or associated with TMAP officials. A survey was not necessary. These persons simply met and decided that the identical drugs being used on adults should also be used on children. There were no studies or clinical trial results whatsoever to support this consensus…
In 1997-98, TMAP, with pharmaceutical industry funding, began working on the Texas Children’s Medication Algorithm Project [TCMAP]. An "Expert Consensus" panel was assembled to determine which drugs would be best for the treatment of mental and emotional problems in children and adolescents. The panel consisted almost exclusively of persons already involved in TMAP or associated with TMAP officials. A survey was not necessary. These persons simply met and decided that the identical drugs being used on adults should also be used on children. There were no studies or clinical trial results whatsoever to support this consensus…
 I almost feel like I ought to make an apology. For the last month, my head has been partially elsewhere – a wedding anniversary of note and a celebration cooked up by our daughters that drew a crowd from our earliest days forward. It’s over now, and things are edging their way back to normal. That picture above was taken by a visitor the morning after a gathering on the back porch over the weekend. I got an email asking if it’s a permanent change, did it have a meaning? I think I put it there because I liked it. It reminded me of the weekend and the coming together of friends from different times in our lives.
I almost feel like I ought to make an apology. For the last month, my head has been partially elsewhere – a wedding anniversary of note and a celebration cooked up by our daughters that drew a crowd from our earliest days forward. It’s over now, and things are edging their way back to normal. That picture above was taken by a visitor the morning after a gathering on the back porch over the weekend. I got an email asking if it’s a permanent change, did it have a meaning? I think I put it there because I liked it. It reminded me of the weekend and the coming together of friends from different times in our lives.  But I can always generate a symbolic meaning in a pinch, and I thought of a couple immediately. First, in this enterprise of trying to clean up the mess made by the academic·pharmaceutical complex, it’s time for a coming together of people from different places and times with a shared purpose rather than a litany of differences.
But I can always generate a symbolic meaning in a pinch, and I thought of a couple immediately. First, in this enterprise of trying to clean up the mess made by the academic·pharmaceutical complex, it’s time for a coming together of people from different places and times with a shared purpose rather than a litany of differences.  I hope that it gets written in all the complexity it deserves. A lot of it is in books already written, but not often read.
I hope that it gets written in all the complexity it deserves. A lot of it is in books already written, but not often read. 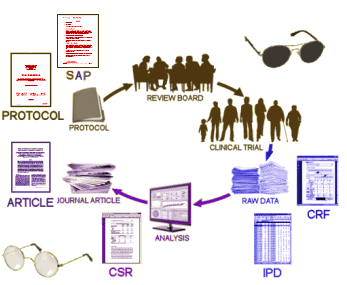
 You’d think as much as I’ve said about these articles already, that I’d shut up about them. But there’s a reason I haven’t. Reading blog posts from a boring old man can’t possibly do strong articles like these justice. They need to be read in person rather than in summary. And I found out that Cosgrove et al is finally available full-text on-line so it can [and should be] read by all. Here are the three articles with their full text links, and a fourth similarly important recent clarifying meta-analysis thrown in for good measure:
You’d think as much as I’ve said about these articles already, that I’d shut up about them. But there’s a reason I haven’t. Reading blog posts from a boring old man can’t possibly do strong articles like these justice. They need to be read in person rather than in summary. And I found out that Cosgrove et al is finally available full-text on-line so it can [and should be] read by all. Here are the three articles with their full text links, and a fourth similarly important recent clarifying meta-analysis thrown in for good measure:
 I recall a time when depression was not so clouded with guild wars or ideology, not so influenced by pharma and insurers. The past is often referred to as a simpler time, but in this case, that wasn’t true. I recall it as a better time, though anything but simpler. It was the right brand of complex in that we were clearer about what we did and didn’t know. For as much as we’ve all had to say about this topic, Ed Shorter, a primo historian of psychiatry, has condensed it down to its essence in this blog post – a response to a new book written by Peter Kramer, author of the 1993 Listening to Prozac:
I recall a time when depression was not so clouded with guild wars or ideology, not so influenced by pharma and insurers. The past is often referred to as a simpler time, but in this case, that wasn’t true. I recall it as a better time, though anything but simpler. It was the right brand of complex in that we were clearer about what we did and didn’t know. For as much as we’ve all had to say about this topic, Ed Shorter, a primo historian of psychiatry, has condensed it down to its essence in this blog post – a response to a new book written by Peter Kramer, author of the 1993 Listening to Prozac: