I guess I ought to start this post by saying what it’s all about, because I doubt I’ll get to the end even in this second post. It’s based on some observations. First, although the direct information on Dan Markingson’s clinical state during his time in the CAFE Trial is spotty, I haven’t read anything that sounds like "better" along the way. His mother was sure he wasn’t improving throughout the whole study. In the month before his suicide, she wrote to Dr. Schulz:
On March 15. 2004 I sent via certified mail a letter addressing my concerns regarding the treatment my son, Dan Markingson, is receiving through the CAFE program. I have not had the courtesy of a reply. I have again left messages for Dr. Olson asking him to point out how he feels Dan is doing better than when hospitalized. I have not received a reply. Actually. I believe the most lucidity I’ve seen Dan in the last six months was when he was first hospitalized, and was on an anti-anxiety medication. He said to me, "Mom. Through all of this you never told me everything would be okay" [I. of course, assured him it would, and it will!]. That was also the only time he indicated any awareness of a problem!…
It sounds to me like he was in the throes of his psychosis the whole time.
Second, the Protocol is clear that
the foremost criteria for discontinuing a subject from the study was lack of improvement on the study drug, and that was a decision to be made by the clinician [see
Protocol p.9].
Third, In his deposition, Dr. Stephen Olson seems absolutely convinced that Dan was not psychotic or deteriorating:
To the best of my knowledge, we thought Dan was taking his medication and it was being monitored after January and February by the staff at Theo House. And in terms of the deterioration, there was no evidence that came to light either before his suicide or after that he was suffering a psychotic decompensation. The only deterioration that we noted was some deterioration in his grooming and other negative symptoms which are manifestations of schizophrenia that do tend to increase over time, but they’re not amenable to treatment with antipsychotic medications, and there was no indication that he had any return of the behavior being influenced by his delusional thinking.
I’m not aware that there are substantial data – you’re implying from rating scales that we’re performing, no, I’m not aware that there is evidence of substantial deterioration.
These three things just don’t go together. There’s a disconnect…
In a tweet about Schulz stepping down, Carl Elliot mentioned a 2011 article that he suggested reviewing in the Minneapolis City Pages. I think he made that recommendation because it has a section on Schulz’s history, and his relationship with
AstraZeneca, maker of
Seroquel® and sponsor of the
CAFE study. But I didn’t make it that far down the page, because it began with some things about the case of Dan Markingson that tapped these still open questions for me. In the first thing I ever read about this case [
The Deadly Corruption of Clinical Trials], there are a number of vignettes and several emails that document the intensity of Dan’s psychosis. This article mentioned by Carl Elliot adds more to the story that I didn’t know:
Is U of M department of psychiatry chair in the pocket of AstraZeneca?
Minneapolis City Pages
By Andy Mannix
Feb 2, 2011
Mary Weiss knew something wasn’t right with her son. Only a year before, Dan Markingson had seemed perfectly normal. But his latest letter from Los Angeles suggested a troubled mind. He claimed he was about to become famous. He was at a crossroads in his life, and would soon have more free time. He even had a big movie premiere in the works. "I knew then that something was wrong," says Weiss. "I knew that there wasn’t a premiere, and when he said he was going to have a lot more free time, I thought he was quitting his job."
Weiss immediately jumped in her car and drove to California. When she arrived, she found her son far worse off than she’d feared. He was talking nonsense and couldn’t be reasoned with. Weiss tried to convince Markingson to come back to Minnesota, where she could look after him. But he had a stipulation: He would only return home if his dead grandmother Daisy told him to. Weiss went to an internet cafe down the street and created an email account under the name "GuardianAngelDaisy." Pretending to be her own deceased mother, she urged Markingson to return to Minnesota. Eventually, he agreed.
He was home for only 10 days before he decided to return to California. Weiss pleaded with him to stay, but he refused. She could either drive him to the airport, or never see him again. Weiss followed him to Los Angeles, where she again tried to urge her son to go back to Minnesota. But this time, his grandmother’s emails weren’t enough. Markingson wanted to talk to a higher authority: Michael the Archangel.
Weiss created another fake email account as Archangel Michael. The two exchanged emails for more than a week before Markingson finally agreed to fly home. Once he was back, Weiss called the South St. Paul police. An officer came to her home to evaluate her son. During the interview, Markingson casually mentioned he would soon be attending a devil-worshipping event in Duluth, and might be ordered to kill people.
That triggered a trip to Fairview University Medical Center, where Markingson was diagnosed with psychosis and placed on a 72-hour hold…
In forty years of practice, I’ve never heard a story like that – the part about the emails to his dead grandmother and the Archangel Michael. I can’t think of any way to read that except as an indicator of how real and how all consuming his psychotic experiences were [and how clever his mother was]. Next came the well known circumstances of his ending up in the CAFE study:
In order to be released, Markingson agreed to a stay of commitment, which would allow him to leave the hospital as long as he followed a treatment plan. The plan involved Markingson enrolling in a study called Comparison of Atypicals in First Episode, or CAFE. The research was sponsored by AstraZeneca, maker of Seroquel, one of the anti-psychotic drugs being investigated. When Weiss found out her son was a human guinea pig, she was furious. She called the hospital and tried to pull her son out of the treatment plan, to no avail. Although Markingson was mentally unfit, he was somehow able to consent to the drug trial.
That Dan was able to keep his psychotic experiences to himself was already well known. He had fooled the police in California on his mother’s first trip. And was apparently on the same path in Minnesota with the police until he "casually" mentioned the devil worshiper thing:
Over the next few months, Markingson’s condition only worsened, Weiss says. His doctor wouldn’t return her calls, so she tried writing a letter to the head of the department, Dr. Charles Schulz. He didn’t reply.
It wasn’t until April 28, after Weiss’s third letter, that she received a cursory response, in which Schulz wrote, "it was not clear to me how you thought the treatment team should deal with this issue." Ten days later, on May 8, Markingson sat in the bathtub of the halfway house where he was staying and stabbed himself to death with a box cutter. "I left this experience smiling!" read the suicide note.
I’ve spent more time than I’d like to admit looking for evidence that Dan improved in the time from his first psychotic communications to his mother until his suicide, and I haven’t found anything. I put what I found in
making sense… and I’ve written people who have seen Dan’s whole journal and say that it’s just more of the same confused psychotic "gibberish." His mother was consistent throughout in saying that what he said while he was in the halfway house didn’t make any sense. Same for the Occupational Therapy and Day Hospital people. I can only conclude that my clinical impression is that he was lost in the throes of his psychosis throughout much of the whole six month period he was in the CAFE study. I can confirm what his mother said [above] in that in his
STRUCTURED CLINICAL INTERVIEW FOR DSM-IV AXIS I DISORDERS he described delusional thoughts, but seemed to have some insight. At that time, he had been on Risperidone 3mg/day for 1½ weeks. In the archives on the
Fear and Loathing site, there’s a collection of
Study Documents which has the SCID on Intake and the PANSS Scale forms [clinician rated] at approximately monthly intervals. They record
no psychotic symptoms. The only comment box filled out is on the last form [04/28/2004 – 10 days before his suicide]:

So in the first post of this series [
under fire I…], I reviewed the evolution of the methodology used in clinical trials of psychoactive drugs from the early days when the outcome came from the subjective reports of clinicians and the trial subjects, to today’s sophisticated rating scales analyzed with supercharged statistics – a move from the subjective to the objective, to evidence-based medicine. In this post, I’ve discussed a case well known to us, a patient in a study for the treatment of a First Episode of a Schizophrenic illness. He was randomized to be treated with Seroquel, and from any perspective I can find here eleven years after the fact, he never responded to that drug even though that wasn’t apparent to his treating physician or in the Clinician rated metrics available to us. His lack of response was certainly apparent to his mother:
I gave Dr Olson examples of Dan’s behavior that lead mc to believe the drug he is on is not beneficial: He still believes he is "bulletproof’ [Dan had told my on several occasions in Los Angeles that he is "bulletproof’, and that I was also when I was with him]. He still believes both that he is an actor [not true], and that he will make a living by giving walking tours of Hollywood. He believes his finances are fine [he owes more than $6,000]. He takes no interest in his appearance [he has been wearing the same pair of khaki pants and cutoff sweatshirt since last November]. Does this sound to you like someone who is getting well?
It is inconceivable to me that any competent clinician could have spent time with Dan and missed the fact that he was quite ill, and that his internal experience was a jumble of disorganized psychotic thinking like the things he wrote in his journal:
Mar 23, 2004: "
world walking, you were at a farm house and we’re getting presents from dogs who had presents fastened in plastic bags to their snouts… in the gloaming and breening, you were thinking of naming it gloaming and greening or gloam-green. That was someone brings a snowslide in summer or midsummer. It has been left behind…" [
Olson 2007 p. 467]
I would expect that to be apparent to a first year psychiatry resident, medical student, or psychology intern after a month or two on the wards. I can only conclude that whoever filled out those clinician-rated PANSS forms was not such a clinician. Not even close. And when Dr. Olson is answering questions in his 2007 deposition, he’s not basing what he says on any meaningful one-to-one engagement, but rather from looking at the PANSS scores or some very superficial contact:
And in terms of the deterioration, there was no evidence that came to light either before his suicide or after that he was suffering a psychotic decompensation…
I’m not aware that there are substantial data – you’re implying from rating scales that we’re performing, no, I’m not aware that there is evidence of substantial deterioration.
So in the next post, I’ll try to stitch together why I think that the evolution of modern Clinical Trial methodology [
under fire I…] and the tragedy of the Dan Markingson case [
this post] are linked together. And we might as well throw in the fact that the prime protocol-defined reason for discontinuing the study was non-response to the study medication. Yet in his six months in the Clinical Trial with no evidence of improvement, he was not removed – even under the escalating pressure on this very point from Dan’s mother, communicated repeatedly to the Study Coordinator [Jeanne Kenney], Dan’s psychiatrist [Stephen Olson], and the Chairman of Psychiatry [Charles Schultz]…
 After graduating from high school in 1960, we scattered to the winds – at least I did. It would be decades before I caught up with the people from those days and heard the stories of how those friends from my earlier life negotiated the tumultuous years that followed. Howard and I reconnected 40 years later on an email thread someone started in the lead-in to a class reunion. A classmate started sending around those hyper-patriotic emails with animated flags that followed 911. The invasion of Iraq was in the air. Howard and I were among the few who opposed it, hardly the majority opinion, and in the next few years, we reconnected in person.
After graduating from high school in 1960, we scattered to the winds – at least I did. It would be decades before I caught up with the people from those days and heard the stories of how those friends from my earlier life negotiated the tumultuous years that followed. Howard and I reconnected 40 years later on an email thread someone started in the lead-in to a class reunion. A classmate started sending around those hyper-patriotic emails with animated flags that followed 911. The invasion of Iraq was in the air. Howard and I were among the few who opposed it, hardly the majority opinion, and in the next few years, we reconnected in person. I don’t need to tell his story because it’s where everything else that matters is – on the Internet [see Howard Moreland on Wikipedia]. The short version is that he left high school pursuing his dream of being a pilot. After a few years as an Air Force pilot flying transports back and forth to Viet Nam, he cut that career short and by 1979, he was a full time anti-nuclear activist and the independent journalist who wrote the article "The H-Bomb Secret: How We Got It, Why We’re Telling It." There was a big First Amendment court battle as the Department of Energy tried to halt publication. It ultimately failed and the article was published in November 1979.
I don’t need to tell his story because it’s where everything else that matters is – on the Internet [see Howard Moreland on Wikipedia]. The short version is that he left high school pursuing his dream of being a pilot. After a few years as an Air Force pilot flying transports back and forth to Viet Nam, he cut that career short and by 1979, he was a full time anti-nuclear activist and the independent journalist who wrote the article "The H-Bomb Secret: How We Got It, Why We’re Telling It." There was a big First Amendment court battle as the Department of Energy tried to halt publication. It ultimately failed and the article was published in November 1979. The article is true to the title – how to get an atomic bomb [fission] to ignite a fusion reaction in the hydrogen fuel in the millionth of a second before everything scatters to the wind – no small feat. The article makes it very clear [it’s a "bank shot"]. Equally interesting is getting the story with no access to classified information [see his slide show]. If it’s not obvious why I’m telling his story, it has to do with the logic used to justify keeping secrets. And I remembered some of the things Howard said when he first told me this story as I was reading Rationale for WHO’s New Position Calling for Prompt Reporting and Public Disclosure of Interventional Clinical Trial Results calling for Data Transparency.
The article is true to the title – how to get an atomic bomb [fission] to ignite a fusion reaction in the hydrogen fuel in the millionth of a second before everything scatters to the wind – no small feat. The article makes it very clear [it’s a "bank shot"]. Equally interesting is getting the story with no access to classified information [see his slide show]. If it’s not obvious why I’m telling his story, it has to do with the logic used to justify keeping secrets. And I remembered some of the things Howard said when he first told me this story as I was reading Rationale for WHO’s New Position Calling for Prompt Reporting and Public Disclosure of Interventional Clinical Trial Results calling for Data Transparency.

 Neuroskeptic, a Neuroscience, Psychology and Psychiatry researcher and
Neuroskeptic, a Neuroscience, Psychology and Psychiatry researcher and  I find this next chapter of GSK USA surrealistic. In January 2011, I read Deirdre Connelly’s speech with amazement. She was the new president of GSK USA, and she was going to shut down the program where Drug Rep’s bonuses were based on the number of prescriptions their territory’s doctors wrote [
I find this next chapter of GSK USA surrealistic. In January 2011, I read Deirdre Connelly’s speech with amazement. She was the new president of GSK USA, and she was going to shut down the program where Drug Rep’s bonuses were based on the number of prescriptions their territory’s doctors wrote [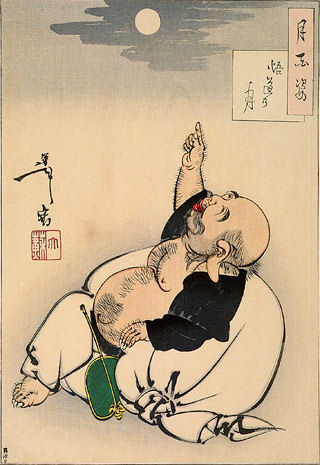 There’s a story of a Zen Master who comes upon a scholar pointing at the moon and lecturing on and on to his students. The Master comments, "that fellow is confusing his finger with the moon." In this case, I’m suggesting that the KOL brand psychiatrists have confused their soft proxies [clinical trials and rating scales] for the actual patients in many instances. In this case, I’m suspicious that they paid attention to the rating scales and not the patient. In other places, they’ve claimed that minor differences have clinical meaning when they don’t. But we often think that all examples portend bad motives, and in some cases they do. But this is an error one can make even without bad motives – by overvaluing secondary information without investigating further.
There’s a story of a Zen Master who comes upon a scholar pointing at the moon and lecturing on and on to his students. The Master comments, "that fellow is confusing his finger with the moon." In this case, I’m suggesting that the KOL brand psychiatrists have confused their soft proxies [clinical trials and rating scales] for the actual patients in many instances. In this case, I’m suspicious that they paid attention to the rating scales and not the patient. In other places, they’ve claimed that minor differences have clinical meaning when they don’t. But we often think that all examples portend bad motives, and in some cases they do. But this is an error one can make even without bad motives – by overvaluing secondary information without investigating further.