Posted on Wednesday 13 August 2014
New York Times: OpinionBy EDWARD LARKIN and IRENE HURFORDAUG 12, 2014Edward Larkin is a second-year medical student at the University of Pennsylvania, where Irene Hurford is an assistant professor in the department of psychiatry.
A few months ago, a patient came to our hospital, seeking help. One of us, Edward, was on the team that treated him. He was pleasant, if slightly withdrawn, and cogent. He was a college graduate in his 20s and had recently been fired from his job as a high school math teacher, because of unexpected absences. He had come to believe that government agents were conspiring against him, and he had taken to living out of a truck and sleeping in different parking lots. By the time he came to us, he was exhausted. A diagnosis became clear: he had . We admitted him to the hospital, and after a few days, with his symptoms under control, we released him. Unfortunately, we prescribed a medication for him that could cause significant, permanent harm, instead of an equally effective drug with milder side effects — all because he was uninsured.
Schizophrenia, which affects 1 percent of the population and emerges in the late teens to early 20s, is deeply misunderstood. People who suffer from it are often suspected of being dangerous, but this is not usually the case, and antipsychotic drugs are very effective. Our patient was exactly the kind of person who, with the right treatment, could have weakened the stigma surrounding schizophrenia. Antipsychotic drugs fall into two classes: the older ones, like Haldol, and newer ones, like Abilify and Latuda. Both classes are equally effective at treating some of the worst symptoms of schizophrenia, specifically the , delusions and paranoia that cause social alienation. [They’re not effective for treating “negative symptoms,” like low motivation].
But the older drugs can cause a multitude of serious side effects, including a potentially devastating one called . This condition involves unsettling, animalistic smacking and wagging of the lips and tongue. At its extreme, it can affect the entire body. It occurs in 20 percent or more of patients who take the drugs long-term, and it tends to start so mildly that patients can’t identify it in time to stop taking the drugs. It is often irreversible.
The newer drugs have lower rates of tardive dyskinesia [estimates vary, but most likely less than half or one-third the risk], although they can cause weight gain and predisposition to , among other side effects. The newest among them, however, have decreased these risks, too. And a 2006 study showed that patients were more likely to keep taking the new drugs than the older ones.
Note from 1boringoldman: This 2006 study is from a proprietary Insurance Claims database with the following financing and ghost? authorship: "This study was supported by AstraZeneca Pharmaceuticals LP. The authors would like to acknowledge the assistance of Anusha Bolonna, PhD [PAREXEL MMS], who provided medical writing assistance on behalf of AstraZeneca." [The article itself makes multiple pair-wise comparisons with no overall ANOVA to justify them, nor did it provide enough information to allow me to accurately estimate the overall ANOVA’s significance].As a result, most psychiatrists prefer the newer drugs, especially for younger people, and they should have been the clear choice for our patient. He didn’t have the luxury of choice, however, because he was uninsured, and he was explicit about the fact that he didn’t have much money to spend on medications. So we had to prescribe him Haldol, which costs about $20 per month, instead of one of the newer drugs, which can cost more than $600 per month. These issues will be amplified as progress is made in discovering the mechanisms of psychiatric disease, as it likely will be, thanks to the billions of dollars that are now going to neuroscience research. We can already see the results of that kind of investment in oncology, where extravagantly expensive specialty drugs are coming on the market. But as we make much-needed progress and develop new, expensive treatments that are clearly superior to old, cheap ones, we have to ask: Will those with the most to gain still receive a lower standard of care?
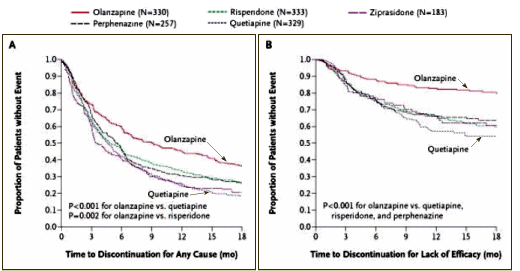
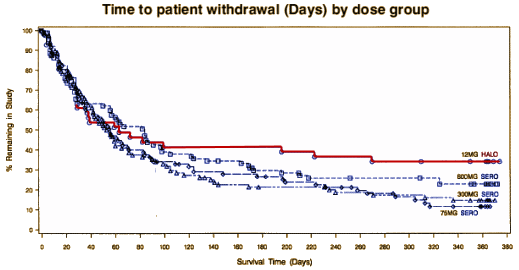
So while his point holds that we aren’t taking good-enough care of Schizophrenic patients, it’s not our using the older medications that’s the problem [in fact, there are generic Atypicals now as available as the first generation antipsychotics]. Our failure is in offering little more than medications to his young patient and vague hopes for some future breakthrough medication that will fix everything.





 But I think, as I said, I came in a lull. It appears that the forces of regulation in the universe have been chasing the Pharmaceutical Industry from its origins in the days of Patent Medicines to the present, only to be periodically thrown off the scent by good behavior after one or another reform movement. But when things settle down, they’re up to their old tricks. We put up with their walks on the wild side because we count on them for new drug development. My own take on all of this is that it’s always a Faustian Bargain guaranteed to come to no good end, and we’d best rethink the whole thing. This may well be an area where capitalism can never work. It is really not a "free enterprise" in that patients, and to some extent doctors, are a captive audiences under the current system. And as for GSK, enough is long past enough…
But I think, as I said, I came in a lull. It appears that the forces of regulation in the universe have been chasing the Pharmaceutical Industry from its origins in the days of Patent Medicines to the present, only to be periodically thrown off the scent by good behavior after one or another reform movement. But when things settle down, they’re up to their old tricks. We put up with their walks on the wild side because we count on them for new drug development. My own take on all of this is that it’s always a Faustian Bargain guaranteed to come to no good end, and we’d best rethink the whole thing. This may well be an area where capitalism can never work. It is really not a "free enterprise" in that patients, and to some extent doctors, are a captive audiences under the current system. And as for GSK, enough is long past enough…
 Dr. Kupfer, et al apologized for lying about COI after being exposed. And in that apology, they didn’t get around to apologizing to Dr. Carroll for their initial nastygram in response to his criticism of their paper [
Dr. Kupfer, et al apologized for lying about COI after being exposed. And in that apology, they didn’t get around to apologizing to Dr. Carroll for their initial nastygram in response to his criticism of their paper [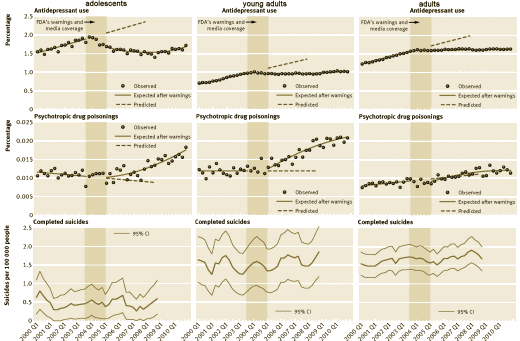
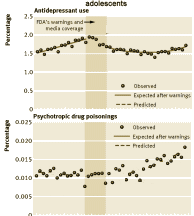 I don’t care to enter that argument because I don’t accept that the graph is related to the question at hand – because it isn’t. Again, end of story. I was tempted to argue with their analysis after removing their imputed lines from the graph. I’m not convinced that a straight line isn’t a better fit than their fancy quadratic quaisi-experimental fitting, particularly if you remove the last data point [that creates something of an optical illusion]. But I realized that line of criticism is just my bias engaging their flawed study. As I said previously in
I don’t care to enter that argument because I don’t accept that the graph is related to the question at hand – because it isn’t. Again, end of story. I was tempted to argue with their analysis after removing their imputed lines from the graph. I’m not convinced that a straight line isn’t a better fit than their fancy quadratic quaisi-experimental fitting, particularly if you remove the last data point [that creates something of an optical illusion]. But I realized that line of criticism is just my bias engaging their flawed study. As I said previously in  Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our
Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our ![Gregor Mendel [1822-1884] Gregor Mendel [1822-1884]](http://1boringoldman.com/images/mendel-1.gif) brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand…
brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand… The Human Genome Project was completed in draft form in 2000, and declared finished in 2003. With that, they left most of us mere mortals in the dust with their volumes and volumes of books filled with Cs, As, Ts, and Gs running on towards the end of time. And so much for pea plants, eye color, dominance, etc. It starts with a gene being "a locatable region of genomic sequence, corresponding to a unit of inheritance, which is associated with regulatory regions, transcribed regions, and or other functional sequence regions" and complexifies geometrically from there. We thought it would be easy, but that was just a dream some of us had [as a kid, I set out to count the stars too].
The Human Genome Project was completed in draft form in 2000, and declared finished in 2003. With that, they left most of us mere mortals in the dust with their volumes and volumes of books filled with Cs, As, Ts, and Gs running on towards the end of time. And so much for pea plants, eye color, dominance, etc. It starts with a gene being "a locatable region of genomic sequence, corresponding to a unit of inheritance, which is associated with regulatory regions, transcribed regions, and or other functional sequence regions" and complexifies geometrically from there. We thought it would be easy, but that was just a dream some of us had [as a kid, I set out to count the stars too].

 I’m not going to bother to summarize what he said in that post. I’ve already done that [
I’m not going to bother to summarize what he said in that post. I’ve already done that [