From 1993 through 1995, Astrazeneca undertook a large, multi-center trial comparing one of the older antipsychotics, Haldol, to several different dose ranges of their new drug, Seroquel [
Study 15]. This was prior to Seroquel’s approval by the FDA. Study 15 didn’t come out like they wanted. The internal memo above is from a high ranking Astrazeneca official praising AZ Physician, Lisa Arvanitis, for her ‘smoke-and-mirrors’ job in trying to discount the actual results [‘smoke-and-mirrors’ in
red]. As it turned out, Astrazeneca "buried" this study along with several others.
4.1.4 Summary of time to withdrawal / time to relapse
There was no statistically significant dose response among SEROQUEL groups in the time to withdrawal from the trial in the intent to treat population, which was the primary efficacy variable. Pairwise comparison showed no statistically significant differences among any treatment group, including haloperidol, in the time to withdrawal from the trial. There was also no statistically significant dose.response among the SEROQUEL groups in the time to withdrawal from the trial for psychotic relapse. Times to psychotic relapse were generally longer in the haloperidol group. Pairwise comparisons of the time to withdrawal for psychotic relapse revealed statistically significant differences between the haloperidol group and each SEROQUEL group. However, there was an imbalance in the reasons for withdrawal from the trial between the SEROQUEL and haloperidol treatment groups with proportionally more patients in the haloperidol group withdrawing for adverse events. Since proportionally more censoring for relapse occurred in the haloperidol treatment group, any contrasts between the haloperidol and SEROQUEL groups for time to withdrawal for psychotic relapse may not reflect true differences in the relapse distributions among the groups and therefore are non.informative. Results of the analysis of the time to withdrawal in the secondary population showed a similar trend as seen in the intent to treat population. The analysis of prognostic variables indicated that only the interaction between treatment groups and the need for neuroleptic medication to be tapered during Segment A were significantly associated with time to withdrawal.
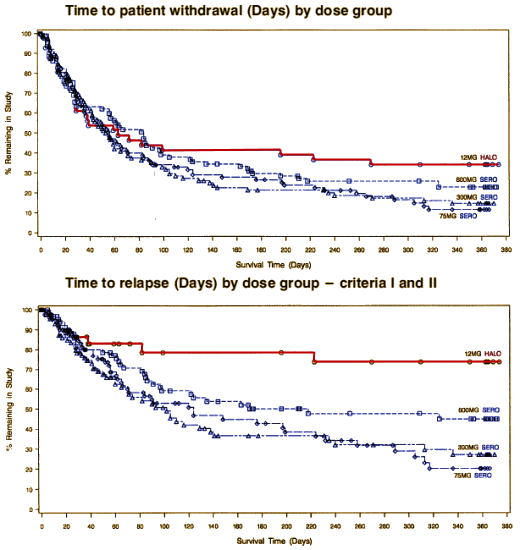
Haldol won the trial
hands down – one of several examples of Astrazeneca suppressing it’s own studies because they didn’t play well with their marketing strategy [
PsychRights has a treasure trove of equally incriminating documents]. As we know, Seroquel went on to be FDA approved with extension for a variety of conditions. It’s now the most lucrative of the Atypical Antipsychotics, themselves the most lucrative drugs in the American pharmacopeia.
Another example of Astrazeneca’s sleight of hand with data was the
CAFE Study. It was planned to compare the response of first-break Schizophrenic patients to the Atypical Antipsychotics –
Seroquel,
Zyprexa, and
Risperdal. According to the
published results: "
CONCLUSIONS: Olanzapine, quetiapine, andrisperidone demonstrated comparable effectiveness in early psychosis patients, as indicated by similar rates of all-cause treatment discontinuation." But there was much more to say about this study [
The Deadly Corruption of Clinical Trials,
"Making a Killing": New Carl Elliott Article in Mother Jones,
Was the CAFE study manipulated by AstraZeneca? Maybe Not,
Comment by Dr. Carroll]. First off, the
CAFE study, financed by Astrazeneca, was in marked contrast to the
CATIE Study, an NIMH funded study, where Seroquel came out on the bottom of the three. Carl Elliot [author of
The Deadly Corruption of Clinical Trials and
White Coat, Black Hat: Adventures on the Dark Side of Medicine] has reported on the tragic suicide of Dan Markingson, one of the patients in the
CAFE Study. Elliot is a bioethicist at the University of Minnesota. After convincingly dissecting why the
CAFE study was strictly a marketing ploy by Astrazeneca, he ends
The Deadly Corruption of Clinical Trials with:
…Yet another problem with the CAFE study is its failure to compare Seroquel to any older antipsychotics. "It’s quite a marketing exercise to put all patients in the CAFE study on atypical antipsychotics," says Dr. Glen Spielmans, an associate professor of psychology at Minnesota’s Metropolitan State University. "It removes the older drugs from the discussion." One reason AstraZeneca may have done this, he suggests, is that Study 15 had already shown Seroquel to be inferior to the older antipsychotic, Haldol.
The bluntest assessment of the study came from Dr. David Healy, a senior psychiatrist at Cardiff University in Wales. Healy is a former consultant to AstraZeneca, among other pharmaceutical companies, and a prominent critic of the industry. "This is a non-study of the worst kind," he said. "It is designed not to pick up a difference between the three drugs. It looks like an entirely marketing-driven exercise."
If these experts are right, then the study in which Dan Markingson committed suicide was not simply a matter of inadequate informed consent, or financial conflicts of interest, or even failure to monitor a subject’s care. The ethical breach was built into the study from the start. It is one thing to ask people to take risks for science, or the common good, or to help other people. It is another thing entirely to ask them to risk their lives for the marketing goals of AstraZeneca.
The account of the case of Dan Markingson is horrific by any account. He was a 26 years old guy who became flagrantly psychotic, threatening his mother’s life and lost in a set of bizarre delusions. In spite of his dangerousness, and in spite of his mother’s protests, he was allowed to volunteer for the CAFE Study. He was put on Seroquel, and even though he did not respond, he was moved to a halfway house where he ultimately killed himself [brutally], in spite of his mother’s desperate attempts to get him out of the study. It was a travesty. He shouldn’t have been allowed to be in the study. He shouldn’t have been released from the hospital. He should’ve been changed to a drug that controlled his symptoms. But, beyond that, he was in a study that shouldn’t have been done in the first place.
Study 15 and the CAFE Study both used as the outcome how long the patients stayed on their medication. That’s a mighty soft criteria for success. But besides that, Dan Markingson was in a halfway house contingent on staying on his medication. If he stopped taking it, he faced being committed back to the hospital, something he didn’t want. What kind of study is that? Had he not suicided, he would have been recorded as a treatment success for Seroquel in spite of remaining dangerously ill!
Buried Studies, Smoke and Mirrors, Jury-Rigged Trials, paying over a billion dollars for lost suits, etc. The reports are all over the place, yet Seroquel, a weak Atypical Antipsychotic with some dangerous side effects, remains a best-selling drug world-wide. And the web-site clinicaltrials.gov currently lists 100 ongoing trials recruiting subjects for studies using Seroquel for a wide variety of conditions, 30 sponsored by Astrazeneca. In spite of the evidence that dates from before the drug was approved to the present, nothing seems able to stop the Pharmaceutical machine that drives this freight train.
All of this story hinges on one simple fact. Every Seroquel pill that’s taken is because some Physician has signed a prescription for it. As much as we might rale about the deceit of the Pharmaceutical Industry and the invasion of Academic Medicine by corporate profit motives, the "gatekeeper" function of Physicians has been corrupted beyond recognition. It seems that any intervention has to start there. I’m beginning to see why so many people who understand what has happened here focus on the Continuing Medical Education [C.M.E.] system. Seroquel has obviously been successfully imprinted on the collective practitioners’ consciousness as safe and effective. Both designations are questionable at best, sometimes dead wrong…


It is a grave concern of mine, that Seroquel XR is currently being studied at UMN and trialed by the same doctor (Schulz) as the one in the CAFE study where Dan Markingson fell victim to this scandalous that never should have been approved for marketing by the FDA (and never should have been recently approved for use in 10 yr olds! w this background).
The FDA has done nothing to protect people from the unethical, corrupt and for-profit marketing tactics used by big pharma such as the Seroquel scandal. This story has a life of its own, with the sex-for-secrets stunt pulled by Wayne MacFadden, the buried study 15 that doctors never saw–and now AstraZeneca is in a huge push trialing the drug up against placebo! for patent extension–sealing the use of the drug for more psych labels is their goal.
I’d like to see a group of doctors speak at an FDA hearing to ask for this drug to be removed from the market. For the sake of children who are going to be innocent victims of a drug with a black box warning for diabetes and in litigation process for that.
PS–another reason this drug is so dangeous now is due to the antidepressant advertising campaign AstraZeneca is promoting. Innocent ppl go to the doctor, walk out w a rx for what they think is an antidepressant and it is in fact a neuroleptic with a black box warning and a scandoulous background. AstraZeneca is promoting this drug heavily for antidepressant use, similar to how Cymbalta by Lilly was re-marketed for back pain, knee pain and fibro…many ppl used it did not know it was an antidepressant and suffered withdrawals.
Besides the drug is abused for street sale, (Seroquel) and abused such as ‘Maq Ball’ Q-ball, ‘Quell’ and snorted in prisons….this is a drug that should be a controlled substance on top of everything else!
[…] indication. Furthermore, they would be required to make all data public, preventing them from hiding data which don’t support a medication’s proposed indication. Finally, this proposal […]