Clozipine was approved in Europe in 1971 as the first Atypical Antipsychotic, but withdrawn after 4 years because of bone marrow toxicity in 5% of patients. It was approved under careful monitoring for use in refractory cases in this country in 1989. The parade of Atypical Antipsychotics derived from Clozapine began in 1993 and continued for ten years, but the craziness seems to me to have started in the early 2000s. We tend to blame PHARMA [specifically Eli Lilly and AstraZeneca] and the participation of the KOLs [Key Opinion Leaders – physicians in high places who became high-priced detail men], but I’m beginning to think that the CROs [Clinical Research Organizations] have deserved a solid seat in this Hall of Shame. It’s a triumvirate – PHARMA, KOLs, CROs – the Clinical Research Industry. And they earned our enmity fair and square.
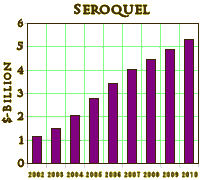
These drugs were introduced as an improvement in the medications used to treat Schizophrenia, but somewhere in the early 2000s, someone got the idea that they could be used for a lot more. We know from subpoenaed documents that as early as early as 1997, AstraZeneca turned over Seroquel research to their Sales and Marketing department, and by 2000 had a strategic plan to expand sales into the Mania and Bipolar arenas. Originally, they were using their KOLs to recommend these indications "off label," but the FDA warned them about the practice. By that time, they had developed a relationship with Parexel, a large CRO. They and the other PHARMAs set out in mid-decade to legitimize their claims for the other indications with a mad flurry of CRO generated Clinical Trials:
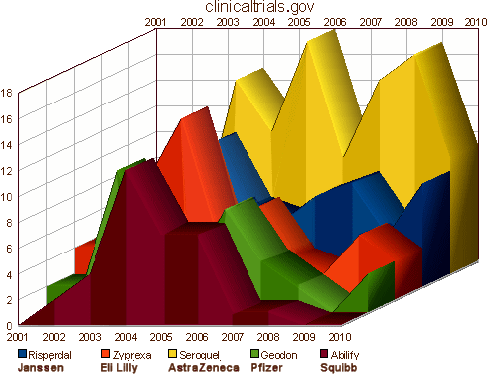
And the campaign for legitimacy paid off:
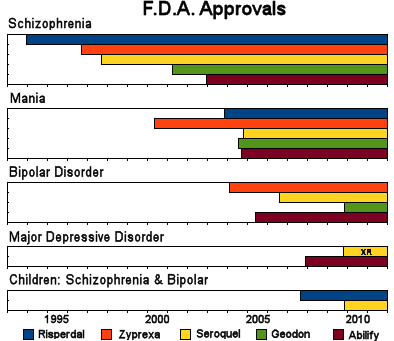
What makes the story worse is that by mid-decade, they knew that these drugs were toxic – caused weight gain, diabetes, EPS, and TD. They were moving into markets where one could not slightly justify the toxicity, much less try to hide it, but they barreled ahead anyway – particularly AstraZeneca [who are still pushing]. Not only did the accumulating indications legitimize their advertising for wider indications, they flooded the medical literature with publications. Many of the articles are largely the production of the CROs from the CRO-generated Clinical Trials – the ones I call CRO-Charts:
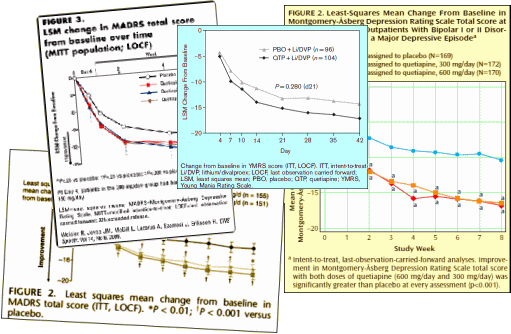
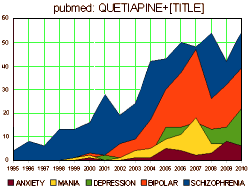 They all look the same. A robust placebo response with a statistically significant but small drug effect. If multiple doses were used, there were no dose response curves. These monotonous studies all use hanky-panky to make the primary data un-retrievable. The burst of articles about these new indications is illustrated on the right using the topics for articles about Seroquel in pubmed, but the other brands were also heavily represented. There’s no way to quantitate how this played out in the speakers bureaus and CME presentations, but it was likely a very prominent part of the message.
They all look the same. A robust placebo response with a statistically significant but small drug effect. If multiple doses were used, there were no dose response curves. These monotonous studies all use hanky-panky to make the primary data un-retrievable. The burst of articles about these new indications is illustrated on the right using the topics for articles about Seroquel in pubmed, but the other brands were also heavily represented. There’s no way to quantitate how this played out in the speakers bureaus and CME presentations, but it was likely a very prominent part of the message.
 So far Eli Lilly has paid $500 M [2007] and $1.2 B [2008] to settle Zyprexa liability suits and a $1.4 B FDA [2009] fine for false advertising. AstraZeneca has paid $198 M, $350 M [2010], and $69 M [2011] to settle suits and a $520 M FDA Fine [2009]. But as has repeatedly been commented on, this is a drop in the bucket compared to their profits from world-wide sales. The standard interpretation is that these seemingly massive pay-outs are simply "the cost of doing business" and easily absorbed by the offending PHARMA manufacturers. The CROs remain largely in the background and are untouched by these penalties levied on their PHARMA employers.
So far Eli Lilly has paid $500 M [2007] and $1.2 B [2008] to settle Zyprexa liability suits and a $1.4 B FDA [2009] fine for false advertising. AstraZeneca has paid $198 M, $350 M [2010], and $69 M [2011] to settle suits and a $520 M FDA Fine [2009]. But as has repeatedly been commented on, this is a drop in the bucket compared to their profits from world-wide sales. The standard interpretation is that these seemingly massive pay-outs are simply "the cost of doing business" and easily absorbed by the offending PHARMA manufacturers. The CROs remain largely in the background and are untouched by these penalties levied on their PHARMA employers.
It’s time to stop simply decrying this rotten state of affairs. It’s time to do something, and the first something is transparency [the modern buzz word]. Paid KOLs are detail men and should be identified and held accountable as such – maybe even registered. Stealth advertising has no place in CME. If it can’t be removed, we’d be better off to suspend CME, at least in Psychiatry. The CRO/CRC Industries are invisible. They should be required to make themselves as visible as PHARMA – pubmed.gov, clinicaltrials.gov, journal articles, etc. Demigod/Ghostwritten articles won’t do. I don’t know how to stop them, but they definitely need stopping. But the thing that I think might go a long way towards substantive reform is to make drug and device patents contingent on the maufacturer’s compliance with FDA guidelines. Instead of just fining the Pharmaceutical Manufacturers when they sin, jerk their patents on the spot – throwing their drug into the world of the generic manufacturers. A patent is a privilege, not a right. And the FDA needs to add the term relevant to efficacy, replacing significant.
What do you think about such entities as the Massachusetts General Hospital’s Psychiatry Academy? MGH/Harvard Medical School has been another center for KOLs and megaprescribing. The “Academy” as far as I can ascertain, is a for-profit enterprise, and I view it as a marketing product. How do you see it?
seems to me we need to broaden the candidate pool for who gets shot at dawn in the morning.
Well I have something to say about MGH. The “Harvard Mafia” ie Joseph Biederman and gang have pocketed MILLIONS into personal bank accts from pharma to the point of investigation by Senator Grassley; Biederman is also investigated for a Risperdal childrens trial protocol breach; Biederman in his depo for a Risperdal lawsuit answered a Q that was exactly what he believes: “I am God”. It’s all documented. He also is (in his own words) responsible for the 4000% increase in childhood bipolar dx the last decade, and likes that label. Good for him. My daughter is disabled because of that direct to doctor KOL influence!
Have you ever read this? Dr Joseph Biederman’s depo ” I am God.”
I haven’t seen that testimony, but would like to.
I actually think I saw a clip of it on Aljazeera on a show they did about mental illness, as I recall. Maybe someone like Stephany can help provide a link to it? I tried and failed. But it was interesting to see and hear it (the testimony) instead of just reading it. To me, the arrogance came hurtling at you off the screen. Narcissistic Personality Disorder, anyone?
Keep in mind–when Sales and Marketing controls the research, they control the dollars. Their only goal is to sell–it’s what they’re measured on, promoted on, get bonuses based on. And that extends to the top. Science and research, to them, is part of sales. When it helps sales, it’s good, and when it harms sales, it’s bad. It’s really that simple. A researcher who finds something that could harm sales, and wants to report it, is a potential menace that could hurt sales. Experts are KOLs-people who can present biased data people will believe, that will increase sales. The KOLs get rewarded as well. The whole system is antithetical to good medicine, and “first do no harm” is a thing of the past.