
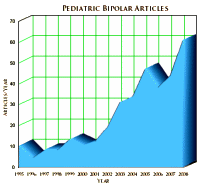
 We’ve already discussed the J&J Center for Pediatric Psychopathology Research at Mass General [harvard for sale…] that started in 2002, and noted the dramatic increase in publication by Biederman’s group at Harvard. But the interest wasn’t just in Boston, the notion of the Bipolar Child caught the interest of the whole international psychiatric community. Beyond the medical articles [right] and conferences, there was an outpouring of books [above] on the subject for the parents of the afflicted. As Dr. Allen Frances discussed later, Pediatric Bipolar Illness took on all the hallmarks of being a "fad." And like many such topics, there were enthusiasts and skeptics, but not many who had no opinion at all.
We’ve already discussed the J&J Center for Pediatric Psychopathology Research at Mass General [harvard for sale…] that started in 2002, and noted the dramatic increase in publication by Biederman’s group at Harvard. But the interest wasn’t just in Boston, the notion of the Bipolar Child caught the interest of the whole international psychiatric community. Beyond the medical articles [right] and conferences, there was an outpouring of books [above] on the subject for the parents of the afflicted. As Dr. Allen Frances discussed later, Pediatric Bipolar Illness took on all the hallmarks of being a "fad." And like many such topics, there were enthusiasts and skeptics, but not many who had no opinion at all.
In 2003, Dr. Biederman got an NIMH Grant [~$100K/yr for 5 years] to host a yearly conference for researchers to get together and share the up-to-date research:
| Project Number: | 1U13MH064077-01A1 | Contact Principal Investigator: | BIEDERMAN, JOSEPH |
| Title: | COLLABORATIVE PEDIATRIC BIPOLAR DISORDER CONFERENCE | Awardee Organization: | MASSACHUSETTS GENERAL HOSPITAL |
|
|
|||
|
DESCRIPTION (provided by applicant): We are proposing a multi-year conference grant which seeks to establish a forum for researchers to pursue collaborative studies of pediatric bipolar disorder. This application was conceived in response to a recent roundtable discussion convened by the NIMH’s Director, Dr. Steve Hyman, in collaboration with the Developmental Psychopathology and Prevention Research Branch and the Child and Adolescent Treatment and Preventive Intervention Research Branch. Despite controversy, the notion that pediatric bipolar disorder is exceedingly rare has been challenged by case reports and emerging research findings that suggest that this disorder may not be rare but, rather, that it is difficult to diagnose. It is also quite clear that, despite debate over nosological issues, many clinicians recognize that a sizable number of children suffer from a severe form of psychopathology associated with extreme irritability, violence, and incapacitation that is highly suggestive of bipolar disorder. Since a sizable clinical population currently exists for which relatively little systematic information is available, efforts that increase the pace and utility of research are desperately needed. Thus, an appropriate mechanism designed to facilitate regular communication among investigators and clinicians is needed as a first step to build collaborative research and guide clinical efforts that will foster a more efficient and streamlined approach to the understanding and treatment of this perplexing disorder. The main aim of the proposed conference grant is to overcome the hurdles to collaboration by establishing yearly conferences among investigators studying pediatric bipolar disorder. Subgoals of these conferences are: (1) to define the boundaries of the bipolar spectrum phenotype and determine if children who technically meet criteria for bipolar disorder actually have this disorder or are affected with another condition.; (2) to standardize data collection methods across different centers to facilitate pooling of diagnostic data and validation of the disorder; (3) to facilitate joint submissions of large collaborative projects that will enable the study of a broad spectrum of scientific questions including genetic, imaging and therapeutic protocols; and (4) to create a mechanism for pooling samples so that potential findings from one group may be cross-validated on pooled data from other groups. Although scientific projects studying pediatric bipolar disorder are likely to be funded in the coming years, these efforts will likely take many years to unfold. This scientific void and ongoing diagnostic and therapeutic uncertainties calls for immediate action to foster contact and dialogue among interested parties in the clinical and scientific community. While the field faces a dearth of information, more and more children and families are being referred to clinics for evaluation and treatment. Thus, steps that increase the identification of children with bipolar spectrum disorder and the development of initial therapeutic approaches to help them is of high clinical, scientific and public health importance. While the proposed conference does not intend to solve all outstanding problems associated with pediatric bipolar disorder, it will provide a forum to begin formulating a solution.
|
|||
Over the course of the years between 2000 and 2008, Biederman’s group reported 9 clinical trials [among their 78 articles]. I included this table because I was surprised at how thin it was – seven small open label trials, one retrospective analysis [of someone else’s Janssen financed double blind study], and only one double blind trial of their own. With all the noise they were making, I would’ve expected more:
| 2008 Risperidone treatment for ADHD in children and adolescents with bipolar disorder [open label, n=31, ages 4-15] Improvement in ADHD symptoms was contingent on improvement in manic symptoms. These results suggest that that treatment with risperidone is associated with tangible but generally modest improvement of symptoms of ADHD in children with bipolar disorder. "This work was supported by grants from Janssen Pharmaceutica (JB), the Stanley Medical Research Institute (JB) and NIH (KO1 MH065523; EM)." |
| 2007 A prospective open-label treatment trial of ziprasidone monotherapy in children and adolescents with bipolar disorder [open label, n=14, 8 week, ages 6-17] …associated with a significant short-term improvement of symptoms of pediatric bipolar disorder. "This study is supported by a grant from Pfizer, Inc." |
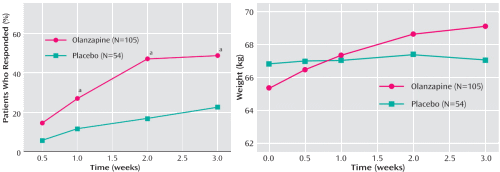
| 2007 Olanzapine versus placebo in the treatment of adolescents with bipolar mania [double-blind, randomized placebo-controlled trial, n=161, 3 week, ages 13-17] Olanzapine was effective in the treatment of bipolar mania in adolescent patients. Patients treated with olanzapine, however, had significantly greater weight gain and increases in the levels of hepatic enzymes, prolactin, fasting glucose, fasting total cholesterol, and uric acid. "Sponsored by Eli Lilly and Company." |
 |
| 2007 An open-label trial of aripiprazole monotherapy in children and adolescents with bipolar disorder [open label, n=15, 8 week] …beneficial in the treatment of mania in youth with bipolar disorder. "This research was supported by Bristol-Myers Squibb." |
| 2006 Risperidone for the treatment of affective symptoms in children with disruptive behavior disorder: a post hoc analysis of data from a 6-week, multicenter, randomized, double-blind, parallel-arm study [double blind, n=110, 6 week] The results of this post hoc analysis of affective symptoms of DBDs using data from a previously published randomized, double-blind clinical comparison of risperidone and placebo in the treatment of children with DBDs and subaverage intelligence suggest that risperidone was effective in treating the factors of explosive irritability; agitated, expansive, grandiose; and depression. "Janssen Pharmaceutica Inc." |
| 2005 Open-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age children [open label, n=31, 8 week, age 4-6] …suggests that treatment with risperidone or olanzapine may result in a rapid reduction of symptoms of mania in preschool children with BPD. Because of substantial residual symptomatology and adverse effects, however, a pressing need exists to identify additional safe and effective treatments for the management of BPD in this high-risk population. "This work was supported by a center grant from the Stanley Medical Research Institute. Aspects of this work were presented at the National Institute of Mental Health (NIMH) Pediatric Bipolar Disorder Conference held in Boston, April 2–3, 2004. The conference was supported in part by NIMH Grant S U13MH64077-03." |
| 2005 An open-label trial of risperidone in children and adolescents with bipolar disorder [open label, n=22, 8 week, age 6-17] Weight increased significantly from baseline and there was a four-fold increase in prolactin levels from baseline. Open-label risperidone treatment was associated with a significant shortterm improvement of symptoms of pediatric bipolar disorder. "This project was supported by a grant from Janssen Pharmaceutica." |
| 2002 An open-label trial of divalproex in children and adolescents with bipolar disorder [open label, n=40, 8 week, age 7-19] This study provides preliminary support for the safety and effectiveness of divalproex in the treatment of bipolar disorder in youths. "Support for this study was provided by Abbott Laboratories." |
| 2001 A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder [open label, n=22, 8 week, age 5-14] Body weight increased significantly over the study. Open-label olanzapine treatment was efficacious and well tolerated in the treatment of acute mania in youths with bipolar disorder "This study was supported by a grant from Eli Lilly and Company and by National Institute of Mental Health Clinical Scientist Award K08-MH-01573 to the primary author." |
Advances in the Neurobiology of Pediatric Bipolar Disorder
by Joseph Biederman and Regina Smith James
Biological Psychiatry 2005 58(7):515-516.…Much of the contemporary diagnostic controversy surrounding pediatric BPD has focused on the diagnostic utility of irritability as a legitimate mood abnormality in the disorder. Two articles deal with this issue. In the first one, Dr. Mick and associates (2005) tackle the thorny issue of irritability in the diagnosis of pediatric BPD. To this end they used data from a large of sample of 274 ADHD children who were administered the Kiddie Schedule for Affective Disorders and Schizophrenia-Epidemiologic Version (KSADS-E) structured diagnostic interview. From this instrument, they identified irritability in three of its modules: Oppositional Defiant Disorder (ODD), Major Depression and Mania. Results showed that while the irritability ascertained from the ODD module was very common in all ADHD subjects, it was the least impairing and did not increase the risk of mood disorders. In contrast the irritability associated with major depression (mad/cranky irritability) was more impairing and was predictive of unipolar depression and the irritability ascertained from the mania module (Super angry/grouchy/cranky irritability) was the most impairing and was predictive of both unipolar depression and bipolar disorder. These results indicate that although irritability is highly heterogeneous, it’s most extreme form is selectively associated with bipolar disorder in children.
In the second article, Wozniak et al (2005) evaluated whether hypothesized cardinal symptoms of euphoria and grandiosity result in differences in clinical correlates in bipolar youth ascertained with no a priori assumptions about cardinal symptoms. They studied 86 youth satisfying DSM-IV criteria for bipolar disorder with and without proposed cardinal symptoms of euphoria and grandiosity. They found that severe irritability was the predominant abnormal mood rather than euphoria in the overwhelming majority of subjects (94% vs. 51%). They also found that among grandiosity was not uniquely overrepresented in youth with mania, nor did the rate of grandiosity differ whether irritability or euphoria was the abnormal mood symptom. Neither symptom profile, patterns of comorbidity nor measures of functioning differed related to the presence or absence of euphoria or grandiosity. These findings support the clinical relevance of severe irritability as the most common presentation of mania in the young…
Lest there be any question about whether the J&J Center for Pediatric Psychopathology Research was a front-end for J&J’s marketing plans, Biederman’s slides erase any doubt:


I would like to thank you, very much for these posts. This one in particular, if anyone can understand this: brought tears to my eyes, of relief and gratitude that the decade of reading and collecting documents, has been brought together in an investigative, smart series. I feel, in a way I can lay down my pen. There is, with the proof of so many articles, abstracts and internal documents a concrete explanation that I saw myself, but always felt no one really understood what I was waving around saying, hey look, did you see how this has been corrupt, planned, marketed? My daughter suffered at the hands of that grand plan and so have countless others.
Thank you, thank you !!!
Great post.
Our son was caught up in Biederman’s ‘Bipolar Juggernaut’ (as reported in Bloomberg, and posted on Pharmalot).
Thank you for all your work in putting this post together.
Duane Sherry, M.S.
discoverandrecover.wordpress.com
The most important “book” for parents and guardians of early-onset bipolar patients — indeed, of any children with mental illness — to read is the Americans with Disabilities Act, or ADA.
There they will learn of the rights afforded such children/young adults under the ADA, and of the steps to take if those rights are violated by others, most notably in the educational arena.
While the rest of us may have to agree to disagree, the young people in question have been granted certain “civil rights” under US law.
So Mickey, when’s the book coming out? I’m serious. Just look at the impact you’ve already made in Duane’s and Stephany’s lives with just this one post. And all of your posts are so airtight they leave me gasping for breath! If you collected and expanded on all your posts into a book-length manuscript concerning the all-out corruption of psychiatry, we may very well have another ANATOMY OF AN EPIDEMIC!
But whatever you decide to do, DON’T STOP. Your work is just too important.
RH,
I agree. The ADA’s “least restrictive” clause is pretty clear.
IMO, it will not be pscyhiatry or even psychology that bring about the paradigm shift needed. It will be the ‘rehabilitation’ community. Some good work is being done at both Temple University – Collaboration on Community Intregration; along with Boston University – Repository of Recovey Resources.
Until we begin to (once again) believe in the human spirit’s ability to overcome we will be stuck in a ‘medical model’ versus a ‘recovery model’…. and until we begin to encourage employment, full-inclusion, Constitutional rights (along with those granted in the ADA), we are stuck.
And it’s time to move forward.
“Rehabilitation’ may be too weak a word… along with ‘recovery’…
Thriving.
We need to encouarge people to thrive!
Beginning with those who have been (mis) dignosed with the most severe “mental illnesses”, and (mis) treated by a failed paradigm of care.
Happy Independence Day to all –
http://discoverandrecover.wordpress.com/freedom
Duane
D and R
We’re not talking about the same things at all.
RH,
My apologies.
Would you clarify?
Duane
[…] they have wreaked on the public, to which this “apology” letter attests. (Thanks to One Boring Old Man via Stephany and for bringing this wrap-up to my […]