There is a persistent assumption that drug choice for the treatment of depression can be based on something other than adverse effects – that some people might preferentially respond to one or another drug or class of antidepressants. While there are allusions that this hypothesized differential response may have etiologic implications, often it is assumed with no reason being given. It is a persistent [and expensive] thought pursued for over a decade [without notable success]:
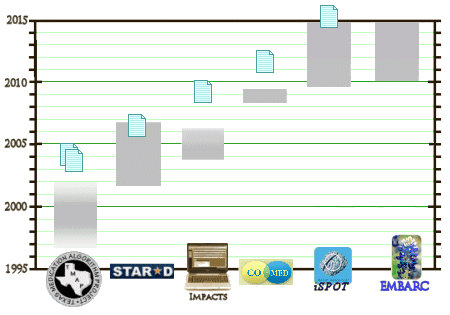
The first three versions were from algorithms that were based on the opinions of their designers as best I could tell when I reviewed them in the Spring [
algorithmic psychiatry – the algorithms…]. Here they are from a distance to show their complexity. Click on the images to see the full-sized versions:
TMAP wasn’t a study [more like a scam];
STAR-D was unintelligible; and
IMPACTS was never even completed. Having failed to get anywhere with arbitrary algorithms, they followed things up with
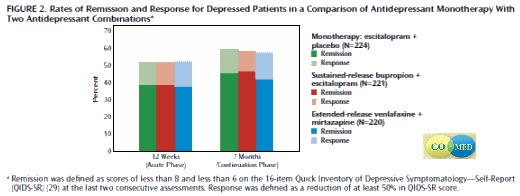
CO-MED – an NIMH funded study using two antidepressant medications at the same time. It was a study where depressed subjects were tried on monotherapy versus two-drugs therapy. There was no Placebo group. The three groups in the study were:
-
Escitalopram [Lexapro] Plus Placebo (N=224)
-
Sustained-Release Bupropion [Wellbutrin SR] Plus Escitalopram [Lexapro] (N=221)
-
Extended-Release Venlafaxine [Effexor XR] Plus Mirtazapine [Remeron] (N=220)
Unlike the algorithm studies,
CO-MED was definitive:
It was a an exuberant failure – no differences found. So having found nothing in their first decade of sequencing and commingling antidepressants in an attempt to increase their efficacy, one might think that they’d move on and come up with something else to think about. While there are a sea of authors’ names on these studies, I’m speaking here of the principles Dr. A.J. Rush and Dr. M.H. Trivedi both at U.T. Southwestern at the time. But some ideas die slowly, it seems.
I know that I perseverate on these particular studies, but it seems to me that rather than apologize, I should be wondering instead why it isn’t an international pastime. These four outings have cost millions [or more]. The TMAP algorithms cost the State of Texas [and other States] an untold amount of money [hopefully revealed in the coming trial in the suit against J&J in November]. STAR*D came in at $35 M and neither IMPACTS nor CO-MED came cheap [all three on the NIMH nickle]. For the last several weeks, I’ve been roaming around the next two offerings [iSPOT and Trivedi’s NIMH study] focusing on their hopes that some genetic bio-markers will be found that will direct treatment. But both studies have other aspects besides just sending samples to the genomics laboratories. Paradoxically, these are the studies that should have come before the ones already mentioned.
Trivedi’s NIMH study [EMBARC]:
This study began as a comparison among
Citalopram,
Bupropion, and
Cognitive Behavior Therapy [
RePORTER], but by the time it was registered as a Clinical Trial [July 25, 2011], it had become a comparison among
Citalopram,
Bupropion, and
Placebo. [
clinicaltrials.gov]. It has the
SMART design motif [
sequential multiple-assignment randomized trial], meaning that non-responders are then switched to the
other treatment. So it’s another drug sequencing scheme. It now has a name and a
website [
EMBARC – Establishing Moderators/Mediators for a Biosignature of Antidepressant Response in Clinical Care]. They plan to "
…assess a comprehensive array of carefully selected clinical (i.e. anxious depression, early life trauma, & gender) and biological (i.e. genetic, neuroimaging, serum, epigenetic & qEEG) moderators and mediators of outcome. Using innovative statistical approaches the identified moderators and mediators will then be used to develop a differential depression treatment response index (DTRI)…
The DTRI will help clinicians match treatments to patients with MDD, resulting in timely selection of treatments best suited for individual patients."
International Study to Predict Optimized Treatment for Depression [iSPOT]:
The
iSPOT design is different. They are comparing
Escitalopram,
Sertraline, and
Venlafaxine-XR [without a
Placebo group]. After phase I, they plan to analyze the many parameters they are measuring and develop biomarker driven algorithms to use in a repeat phase II study. Their Clinical Trial is underway [
clinicaltrials.gov], registered on June 6, 2008.
So this time around, they’re measuring any and everything possible to try to find an algorithm that might work [at least the algorithms might be coming from data instead of just their opinions]. The
iSPOT study is even brave enough to try out whatever they come up with. Trivedi is still in the driver’s seat of
EMBARC. This time, Rush is on the sidelines [in
iSPOT] with this assignment – "
AJR is Chair of the iSPOT-D Publication Committee" [a fitting assignment since he got 100+ publications out of STAR*D] teamed with his STAR*D sidekick, "
We gratefully acknowledge the editorial support of Jon Kilner, MS, MA (Pittsburgh, PA, USA)."
Will these two studies find something of enduring value? I really doubt it [with the genetics part, I double-dog-doubt it for reasons I’ve run into the ground previously]. Will they finally lead us to accept the null hypothesis that there’s no extra mileage to be gotten out of the antidepressants than we already know? I certainly hope so. The net amount of treasure spent testing them in trials, against each other, in tandem, in combination, with augmentation, etc. would run a small country…
The net amount of treasure spent testing them in trials, against each other, in tandem, in combination, with augmentation, etc. would run a small country…
maybe they are running their small kingdom…
A.L.