National Trends in the Antipsychotic Treatment of Psychiatric Outpatients With Anxiety Disorders
by Jonathan S. Comer, Ramin Mojtabai, and Mark Olfson
American Journal of Psychiatry 2011 168:1057-1065.
Objective: The purpose of the present study was to examine patterns and recent trends in the antipsychotic medication treatment of anxiety disorders among visits to office-based psychiatrists in the United States.
Method: Annual data from the 1996–2007 National Ambulatory Medical Care Survey were analyzed to examine the patterns and trends in antipsychotic medication treatment within a nationally representative sample of 4,166 visits to office-based psychiatrists in which an anxiety disorder was diagnosed.
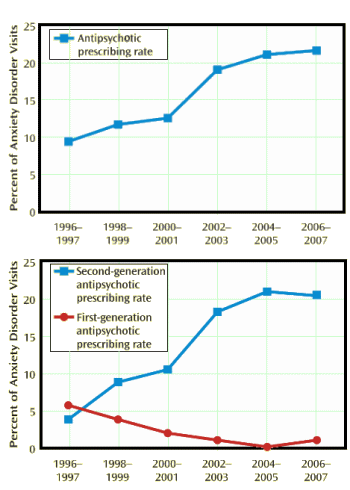
Results: Across the 12-year period, antipsychotic prescriptions in visits for anxiety disorders increased from 10.6% (1996–1999) to 21.3% (2004–2007). Over the study period, the largest increase in antipsychotic prescribing occurred among new patient visits. Antipsychotic prescribing also significantly increased among privately insured visits and visits in which neither antidepressants nor sedative/hypnotics were prescribed. Among the common anxiety disorder diagnoses, the largest increase in antipsychotic medication treatment was observed in visits for panic disorder. Antipsychotic prescribing rose from 6.9% (1996–1999) to 14.5% (2004–2007) among visits for anxiety disorders in which there were no co-occurring diagnoses with an indication approved by the Food and Drug Administration for antipsychotic medications.
Conclusions: Although little is known about their effectiveness for anxiety disorders, antipsychotic medications are becoming increasingly prescribed to psychiatric outpatients with these disorders.
Anxiety Disorders and Antipsychotic Drugs: A Pressing Need for More Research
by Alan Breier, M.D.
American Journal of Psychiatry 2011 168:1012-1014.
Anxiety disorders are a common, heterogeneous group of illnesses marked by varied clinical presentations, ranging from relatively moderate symptom levels to severe functional impairment and profound disability. Pharmacotherapy, often coupled with behavioral treatments, is a standard approach to treating anxiety disorders. First-line pharmacological treatments for the majority of anxiety disorders are the selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors. Sedative-hypnotics are commonly employed for anxiety disorders but are frequently reserved for short durations and targeted symptoms because of the potential for abuse, dependency, withdrawal reactions, and cognitive impairment. The role for antipsychotic drugs in the treatment of anxiety disorders, however, is less clear because of the relative paucity of well-designed trials to assess their safety, efficacy, and effectiveness…Another factor that may have contributed to the rise in antipsychotic prescriptions during the time frame of the study’s data collection is widespread and inappropriate marketing practices. While psychiatrists are entitled to use medications for nonapproved conditions such as anxiety disorders, U.S. Food and Drug Administration regulations forbid pharmaceutical companies from promoting the use of medications for nonapproved conditions — yet there is documentation that the marketing of second-generation antipsychotics for nonapproved conditions took place during the period of data collection for this study.
The use of antipsychotics for anxiety disorders is not approved by the FDA. This point raises a dilemma for clinicians, patients, and payers as to what steps to take when FDA-approved medications fail to deliver adequate clinical outcomes. This scenario is commonly faced in the field of psychopharmacology as prescribers must decide to go off-label and, for example, use higher than approved doses, add combination therapy, or switch to other medications that may not be FDA approved… For clinical conditions for which there are no FDA-approved treatments or for which FDA-approved treatments have failed and there is still a clinical need for treatment, it is incumbent on the academic and clinical fields to provide guidance in the form of expert consensus guidelines and treatment algorithms that are supported by comprehensive reviews of the clinical literature…
Perhaps the greatest value of the Comer et al. study is that it calls attention to the pressing need for more research to evaluate antipsychotics for use in patients with anxiety disorders given the high and growing use of these agents in this patient group. We need both well-controlled randomized clinical trials that evaluate the safety and efficacy of antipsychotics for anxiety disorders and large-scale effectiveness studies that determine outcomes in real-world practice settings. Key questions include the following: Do antipsychotics, either as monotherapy or in combination with first-line agents, provide a favorable safety/efficacy profile for anxiety disorders? Is there a unique role for low-dosage antipsychotics in the treatment of anxiety disorders? Do the second-generation antipsychotics that have the most favorable safety profile [the lowest rates of weight gain, metabolic complications, and motor side effects] offer clinical advantages compared with other antipsychotics for patients suffering from anxiety disorders? What treatment approaches are most effective for treatment-refractory anxiety disorders? Given the heterogeneity of anxiety disorders, which individual disorders and symptom complexes, if any, are best suited for antipsychotic therapy? Once the clinical trial database of antipsychotics for anxiety disorders is enriched, clinicians will be able to make rational, evidenced-based decisions about their clinical use for this important group of disorders.
Footnotes:
Dr. Breier has served on advisory boards or as a consultant for Amgen, MedAvante, Takeda, and Teva, and he is a former employee of Eli Lilly. Dr. Freedman has reviewed this editorial and found no evidence of influence from these relationships.
The drug maker Eli Lilly has engaged in a decade-long effort to play down the health risks of Zyprexa, its best-selling medication for schizophrenia, according to hundreds of internal Lilly documents and e-mail messages among top company managers. The documents, given to The Times by a lawyer representing mentally ill patients, show that Lilly executives kept important information from doctors about Zyprexa’s links to obesity and its tendency to raise blood sugar — both known risk factors for diabetes. Lilly’s own published data, which it told its sales representatives to play down in conversations with doctors, has shown that 30 percent of patients taking Zyprexa gain 22 pounds or more after a year on the drug, and some patients have reported gaining 100 pounds or more…
“Olanzapine-associated weight gain and possible hyperglycemia is a major threat to the long-term success of this critically important molecule,” Dr. Alan Breier wrote in a November 1999 e-mail message to two-dozen Lilly employees that announced the formation of an “executive steering committee for olanzapine-associated weight changes and hyperglycemia.” Hyperglycemia is high blood sugar. At the time Dr. Breier, who is now Lilly’s chief medical officer, was the chief scientist on the Zyprexa program…
The documents were collected as part of lawsuits on behalf of mentally ill patients against the company. Last year, Lilly agreed to pay $750 million to settle suits by 8,000 people who claimed they developed diabetes or other medical problems after taking Zyprexa. Thousands more suits against the company are pending…
The documents — which include e-mail, marketing material, sales projections and scientific reports — are replete with references to Zyprexa’s importance to Lilly’s future and the need to keep concerns about diabetes and obesity from hurting sales…
Lilly Announces Changes in Key Leadership Roles
Lechleiter Aims to Reduce Bureaucracy, Streamline Decision Making
PRNewswire – FirstCall via COMTEX News Network
May 21, 2008
INDIANAPOLIS — Eli Lilly and Company (NYSE: LLY) today announced changes across its executive leadership structure as the company continues to position itself to compete and win in a challenging business environment."Earlier this year I asked each of our senior leaders to look closely at their respective areas to determine if they were optimally structured to meet the demands of our business and our company goals," said John C. Lechleiter, Ph.D., who assumed the role of Lilly president and chief executive officer on April 1, 2008. "As a result of this thorough review, we are implementing a number of changes that will minimize bureaucracy by reducing the number of layers of management, will speed decision-making by clarifying roles and accountabilities, and will allow us to respond more quickly to critical business needs"…
Changes in management structure and key leadership roles across several areas of the company have recently been announced to employees, with most changes becoming effective during the second quarter. Highlights of the executive level changes include the following:
The executive leadership structure of the company’s research and development organization, Lilly Research Laboratories (LRL), will undergo changes designed to reduce bureaucracy and clarify accountabilities. Two senior leaders in the LRL organization have announced their intent to depart from the company, which has also led to changes in the management structure…
Alan Breier, M.D., vice president of medical and chief medical officer, has been appointed full professor in the department of psychiatry at the Indiana University School of Medicine after an 11-year career at Lilly. Breier, who joined Lilly in 1997 from the National Institutes of Health, has published more than 235 scientific articles in areas related to the cause and treatment of severe mental illnesses, and last year was honored by the American Psychiatric Association for excellence in medical student education. Breier has held a variety of leadership roles at Lilly on the Zyprexa(R) product team as well as in medical, and has served as the chief medical officer since 2003…
 Eli Lilly and Company Agrees to Pay $1.415 Billion to Resolve Allegations of Off-label Promotion of Zyprexa
Eli Lilly and Company Agrees to Pay $1.415 Billion to Resolve Allegations of Off-label Promotion of Zyprexa
$515 Million Criminal Fine Is Largest Individual Corporate Criminal Fine in History; Civil Settlement up to $800 Million
January 15, 2009
American pharmaceutical giant Eli Lilly and Company today agreed to plead guilty and pay $1.415 billion for promoting its drug Zyprexa for uses not approved by the Food and Drug Administration [FDA], the Department of Justice announced today. This resolution includes a criminal fine of $515 million, the largest ever in a health care case, and the largest criminal fine for an individual corporation ever imposed in a United States criminal prosecution of any kind. Eli Lilly will also pay up to $800 million in a civil settlement with the federal government and the states. Eli Lilly agreed to enter a global resolution with the United States to resolve criminal and civil allegations that it promoted its antipsychotic drug Zyprexa for uses not approved by the FDA, the Department said…
There are lots of times I wonder about how people can say/write things with a “straight face”.
Maybe he should go to Vegas w the rest of them, since he knows so much about Viva Zyprexa! campaign and the illegal marketing of the drug…. he could talk abt off label use of antipsychotics and the dangers of it for patients or the gains for pharma as patent extenders.
The foxes are guarding the henhouse…