Antidepressant discontinuation syndromes [full text on-line]
by Haddad PM
Drug Safety 2001 24(3):183-97.
Discontinuation symptoms are recognised with tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs) and miscellaneous antidepressants. A wide variety of symptoms have been described, differing somewhat between antidepressant classes, and several symptom clusters or discontinuation syndromes appear to exist. A common feature is onset within a few days of stopping the antidepressant or, less commonly, reducing the dosage. Discontinuation syndromes are clinically relevant as they are common, can cause significant morbidity, can be misdiagnosed leading to inappropriate treatment and can adversely effect future antidepressant compliance. Preventative strategies include tapering antidepressants prior to stoppage and educating patients and healthcare professionals to ensure that antidepressants are taken consistently and not stopped abruptly. Most reactions are mild and short-lived and require no treatment other than patient reassurance. Severe cases can be treated symptomatically or the antidepressant can be reinstated before being gradually withdrawn. Reinstatement usually leads to symptom resolution within 24 hours. Some individuals require very conservative tapering schedules to prevent the re-emergence of symptoms. With SSRIs and venlafaxine another strategy to consider is switching to fluoxetine, which may suppress the discontinuation symptoms, but which has little tendency to cause such symptoms itself. Neonatal discontinuation symptoms can follow maternal use of antidepressants during pregnancy and possibly breast feeding. The patient and doctor must take this into consideration when making prescribing decisions. Discontinuation symptoms have received little systematic study with the result that most of the recommendations made here are based on anecdotal data or expert opinion. Research is needed to provide a firm evidence base for future recommendations.
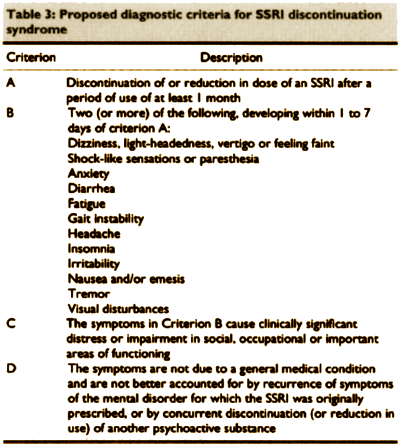
Selective serotonin reuptake inhibitor discontinuation syndrome:
proposed diagnostic criteria
by Kathy Black; Cathy Shea; Serdar Dursun; and Stanley Kutcher
Journal of Psychiatry and Neuroscience 2000 25(3):255-261.
Objective: To establish specific criteria by which selective serotonin reuptake inhibitor (SSRI) discontinuation syndrome may be identified.
Data sources: MEDLINE and PSYCHLIT databases were searched for case reports published from 1986 to 1997 inclusive, and references of relevant articles were also searched.
Study selection: Forty-six case reports of symptoms following the discontinuation of fluoxetine, fluvoxamine, paroxetine or sertraline were selected. Three studies of SSRI discontinuation were also reviewed.
Data extraction: Demographic and treatment information, as well as the timing, duration, number, nature and frequency of dicontinuation symptoms.
Data synthesis: Paroxetine was most frequently implicated. The drug had been tapered in half of the cases. In some cases, symptom onset began during taper, whereas, in most cases, symptoms began within I to 3 days of drug discontinuation. Fifty-three different symptoms were reported, with dizziness being the most common. Other common symptoms were nausea or emesis, fatigue, headache, gait instability and insomnia. Shock-like sensations, paresthesia and visual disturbances were the most rare. Without intervention, symptoms persisted for more than a week in half of the cases. In cases in which the SSRI was restarted, symptoms resolved within 72 hours. In some cases, withdrawal symptoms recurred when the same SSRI was again discontinued.
Conclusions: Findings were used to construct diagnostic criteria for the SSRI discontinuation syndrome.
Withdrawal from paroxetine can be severe, warns FDA
by Alison Tonks
British Medical Journal 2002 324:260.GlaxoSmithKline, a leading drugs manufacturer, was last week forced to admit that paroxetine, a widely prescribed antidepressant and the company’s best selling drug, can cause severe withdrawal symptoms when stopped. The Food and Drug Administration in the United States published a new product warning about the drug, and in the same week the International Federation of Pharmaceutical Manufacturers Associations declared the company guilty of misleading the public about paroxetine on US television a year ago.
“This drug has been promoted for years as safe and easy to discontinue,” said Charles Medawar, head of Social Audit, a consumer research group specialising in medicines policy. “The fact that it can cause intolerable withdrawal symptoms of the kind that could lead to dependence is enormously important to patients, doctors, investors, and the company. “GlaxoSmithKline has evaded the issue since it was granted a licence for paroxetine over 10 years ago, and the drug has become a blockbuster for them, generating about a tenth of their entire revenue. The company has been promoting paroxetine directly to consumers as ‘non-habit forming’ for far too long.”
Mr. Medawar lodged a complaint a year ago after a spokesman from GlaxoWellcome, then a UK company, described withdrawal symptoms with paroxetine as “very rare” during an appearance on an American television network. The spokesman added “[withdrawal] occurs in only two out of every 1000 patients … Even then the symptoms are mild and short lived.” In fact, withdrawal symptoms such as bad dreams, paraesthesia, and dizziness occur in up to 7% of patients, according to the new product information. The warning also mentions anecdotal reports of agitation, sweating, and nausea and tells doctors to consider restarting treatment if symptoms become intolerable. The complaint was originally dismissed but went to appeal. On 18 January the International Federation of Pharmaceutical Manufacturers Associations announced that GlaxoSmithKline had breached two of the industry’s codes of practice. The federation ruled that the spokesman’s comments were promotional and were wrong.
Dr. Peter Haddad, consultant psychiatrist for Salford’s Mental Health Service NHS Trust, welcomed the FDA’s safety warning. He said: “Withdrawal side effects from antidepressants are far commoner than many people realise, and there’s evidence that paroxetine has one of the highest rates. In most cases the symptoms are mild, but in a minority they are severe and prolonged—and treatable only by restarting the drug. There is also the danger of misdiagnosis and inappropriate investigation. Severe dizziness can easily look like labyrinthitis. Patients should be warned not to stop taking their antidepressants suddenly, and doctors should taper the dose at the end of treatment, keeping a close watch for withdrawal symptoms,” Dr Haddad added. He also called for discontinuation problems to be thoroughly assessed before new antidepressant drugs are licensed. “This is a seriously under-researched area. There’s no good evidence to help doctors get the dosing right as patients come off treatment. It’s still a matter of trial and error.”
What the criteria leave out because it’s hard to describe is a not-me feeling and something more like profound despair than sadness or depression. It’s hard for people experiencing these symptoms to accept that they can all be "just from not taking medicine." That is often mirrored by their treating physicians who think this is either a new mental illness or a return of an old one – compounding the problem. Restarting the medication eliminates the symptoms in 24-72 hour and may be necessary for treatment, but also to reassure patient and physician alike of the diagnosis. The universal recommendation is a very slow taper – using the liquid form of the medication if necessary to hit the increments. Some use changing the patient over to a long acting SSRI like Prozac, then withdrawing the medication as Prozac withdrawal is either absent or more benign. Apparently ancillary anxiolytics are symptomatically helpful to many. This can be a simple or prolonged process – varying from person to person. Withdrawal syndromes are reported with all of the SSRIs, but I’ve only personally seen it happen with Paxil and Effexor.
I’m in an interesting situation, and have been thinking about this a lot the past few days. I’m on venlafaxine (effexor) and have been for years. I used to be able to miss a dose here or there — even for days at a time — without ill effects. About a couple of years ago, though, I’ve developed withdrawal symptoms, the most debilitating being severe vertigo. So, I’ve gotten much better at taking it. But I’m in England right now, and will be for two more weeks. But I’ve only got a week’s left of my prescription (my insurance will only pay for one month at a time). I had arranged for my next month’s prescription to be sent to me — but I didn’t know that the US postal service wouldn’t let my friend mail it! So – now I’m facing an involuntary tapering unless I can get the prescription filled here. I always wanted to know if the vertigo would go away — guess I might just have to find out.
Just seems basic to me that if you have trouble stopping any drug, that means it’s addictive, just like nicotine.But I wonder how many patients are told, “this might help you for a while, but you will have a devil of a time stopping it when you want to”. If they are told this, how many decide to take it anyway? And wasn’t the “conventional wisdom” formerly something about when you start your patients on it, you have to tell them it will take 4-6 weeks to be effective? So, are these two things contradictory or is that just my warped thinking? The suicides of patients who were taking medication used to be attributed to “they hadn’t been on it long enough to work?”. Seems to me there is lots of “talking out of both sides of our mouth”.
As an FYI, I became suicidal from a cold turkey of Prozac and Ritalin. Of course, none of the psychiatrists that I saw recognized what was happening which was several years ago.
It sounds like from what you’re saying that nothing much has changed.
You said,
“Why am I writing about this? Because I’ve been called about two cases this month. In both cases, the diagnosis was missed, or at least minimized. In both cases, I knew the treating physician – both solid citizens.”
I mean no disrespect to these physicians but most of your colleagues in my opinion see everything through the eyes of the mental illness label. So as long as you have tunnel vision, of course, you are going to miss withdrawal issues.
I just get the feeling that because you don’t have tunnel vision, Mickey, that even if you hadn’t seen a withdrawal case early in your career, you would have realized this was going on in these two cases.
Regarding withdrawal issues, many people, including me, have had to taper off of meds very slowly at 10% of current dose every 4 weeks. This site, http://survivingantidepressants.org/index.php?/index is a great source of support and is not linked to any commercial interest. It depends on donations.
Thanks for the great work you do.
I forgot to mention that the cold turkey of Prozac and Ritalin was done by one of the psychiatrists I saw and was not my idea.
Haddad 2001: “Most reactions are mild and short-lived and require no treatment other than patient reassurance. Severe cases can be treated symptomatically or the antidepressant can be reinstated before being gradually withdrawn. Reinstatement usually leads to symptom resolution within 24 hours. Some individuals require very conservative tapering schedules to prevent the re-emergence of symptoms.”
Dr. Mickey, thank you for this. One might suspect you have been reading my site about antidepressant withdrawal. There’s a large journal article section at http://tinyurl.com/3z6e2sx
Eliding the serious downside outlined by Haddad in 2001, psychiatry has since published many, many articles emphasizing that withdrawal symptoms are mild and last only a few weeks (thousands of Web posts now contradict this).
Since 2001, Haddad himself has participated in defining withdrawal syndrome categorically as lasting a couple of weeks, see 2006 Shelton et al http://tinyurl.com/3hngcy7 — M. Fava and Schatzberg also were involved, although these experts knew then (and everyone else knows now ) this is wrong, wrong, wrong.
(The J Clin Psychiatry supplement hosting this paper was sponsored by Wyeth, manufacturer of Effexor, a prime offender for withdrawal syndrome.)
Even psychiatrists typically ignore severe cases of withdrawal and no one has ever gotten the “very conservative tapering” part, about which to this very day no protocols have been published.
For lack of very conservative tapering — some sensitive individuals can tolerate reductions of only a fraction of a milligram per month — long-term injuries have resulted, with patients suffering from withdrawal syndrome for months or years.
At the time 2006 Shelton went to press, Shelton sent me this correspondence acknowledging prolonged withdrawal syndrome: http://tinyurl.com/3rgyfhw
The whitewashing of withdrawal has, however, made doctors complacent about a serious adverse effect and allowed the prescription of antidepressants to proceed unimpeded. It’s also caused millions of people to be maintained on antidepressants unnecessarily for years, as withdrawal syndrome is so frequently misdiagnosed as relapse. (Maintenance is where the pharma profit is.)
Although documentation of antidepressant withdrawal syndrome is extensive, the DSM committee repeatedly has rejected a diagnosis for it, although one exists for benzo withdrawal syndrome.
Misdiagnosis of withdrawal for relapse is so widespread it has contaminated all studies of antidepressant efficacy; none contains protocols to identify withdrawal.
Although withdrawal studies show frequency to be 20%-60%, not a single case of withdrawal appears in any efficacy study; they were all counted as relapse. If they had been properly identified, it’s likely the paper-thin margins of statistical significance found for efficacy would go negative.
The contaminated statistics for efficacy reverberate through the entire body of publication on antidepressants. Garbage in, garbage out.
Gigantic coverup or research gap? You be the judge.