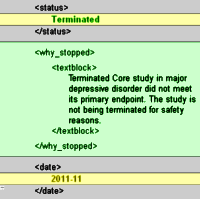
 "Agomelatine is unique in that it is a selective agonist at MT1 and MT2 receptors and an antagonist at 5-HT2B and 5-HT." In these days of the empty pipeline for new CNS drugs, it’s not surprising that people are looking around for novel chemicals hoping for a new blockbuster drug for depression. The paper in the Lancet mentioned in my last post [of sound and fury…] gives a long rationale for trying melatonin and some of its derivatives because of their effect on the sleep cycle. There have been twelve US Clinical Trials of Agomelatine, one recently terminated in November 2011 [right]. The regulatory agency approval history for Agomelatine has been chequered at best:
"Agomelatine is unique in that it is a selective agonist at MT1 and MT2 receptors and an antagonist at 5-HT2B and 5-HT." In these days of the empty pipeline for new CNS drugs, it’s not surprising that people are looking around for novel chemicals hoping for a new blockbuster drug for depression. The paper in the Lancet mentioned in my last post [of sound and fury…] gives a long rationale for trying melatonin and some of its derivatives because of their effect on the sleep cycle. There have been twelve US Clinical Trials of Agomelatine, one recently terminated in November 2011 [right]. The regulatory agency approval history for Agomelatine has been chequered at best:Agomelatine was discovered and developed by the European pharmaceutical company Servier Laboratories Ltd. Servier continued to develop the drug and conduct phase III trials in the European Union. In March 2005 Servier submitted agomelatine to the European Medicines Agency [EMEA] under the trade names Valdoxan and Thymanax. On 27 July 2006 the Committee for Medical Products for Human Use [CHMP] of the EMEA recommended a refusal of the marketing authorisation of Valdoxan/Thymanax [agomelatine]. The major concern was that efficacy had not been sufficiently shown. The CHMP had no special concerns about side effects. In September 2007, Servier submitted a new marketing application for Valdoxan [agomelatine] to the EMEA. On 20 November 2008, Valdoxan was given a positive opinion, with restrictions, by the EMEA, and was subsequently given marketing authorisation in the European Union on 20 February 2009. Release dates in the individual countries of the EU were dependent on marketing arrangements. In March 2006, Servier announced it had sold the rights to market agomelatine in the United States to Novartis. It was undergoing several phase III clinical trials in the US, and until October 2011 Novartis listed the drug as scheduled for submission to the FDA no earlier than 2012. However, the development for the US market was discontinued in October 2011, when the results from the last of those trials became available. It is currently sold in Australia under the Valdoxan trade name.
One of Australia’s most high-profile psychiatrists has come under fire for "overstating" the benefits of a new antidepressant that critics say is ineffective and potentially unsafe. A series of letters to the Lancet Friday accused Professor Ian Hickie of promoting the strength of evidence on agomelatine [Valdoxan], while downplaying safety concerns. They also took aim at the drug company ties of Professor Hickie and colleague Associate Professor Naomi Rogers, suggesting these had contributed to their "subjective" and "inappropriate" appraisal of the drug.Professors Hickie and Rogers, who rejected the claims, had recently authored a Lancet review on the new class of melatonin-based antidepressants, focusing on agomelatine. They said the drug had "clinically significant" effects, with similar short-term efficacy to venlafaxine, fluoxetine, and sertraline. They concluded its favourable safety profile and positive effects on circadian function meant it "might occupy a unique place" in the management of some patients.
However, their article was dissected by six scathing letters to the journal. Several suggested the data had been cherry-picked and not systematically appraised. In one letter, French doctors said that half the placebo-controlled trials of agomelatine had been negative, and others "too weak" to draw conclusions on efficacy. In another, a US psychiatrist said the rare risk of liver toxicity with agomelatine – "a unique safety issue among antidepressant drugs"- was not highighted in published studies.
Professor Jon Jureidini and Melissa Raven, Adelaide-based members of Healthy Skepticism, said in a third letter the article’s summary contained "unjustified and misleading conclusions", and raised concerns over possible conflicts of interest. "The Lancet‘s publication of this flawed paper will undoubtedly validate marketing of Valdoxan, and we are curious to see how many paid Valdoxan advertisements will be published in Elsevier journals," they wrote. However, Professors Hickie and Rogers stood by their review, saying it had been "balanced and independent". Many of the issues, such as the challenge of demonstrating clear efficacy over placebo, were common to all antidepressants, they said, and changes in liver function tests were well recognised with agomelatine, and reflected in monitoring recommendations. They said their paper had been commissioned by the Lancet, was neither initiated nor supported financially by Valdoxan manufacturer Servier, and expressed only the authors’ opinions. Agomelatine is available on private script in Australia, having twice been rejected for PBS listing.
[1] Tomorrow, we are very heavily criticised for publishing a review on melatonin-based drugs for depression. Biased and overstated, say many.
[2] The bias in this paper is very disturbing – it might be fine to argue your case in a Viewpoint or letter. But…
[3] …this paper purported to be an unbiased review of a new drug class. Peer review improved it, yet not enough.
[4] As troubling is the fact that one author took part in speaking engagements for the company making one of these drugs.
[5] It is this kind of complicity that damages any hopes of a positive partnership between medicine and industry.
-
"Why did Ian Hickie, a prominent Professor of Psychiatry in Sydney Australia sign on to this industry-generated, inaccurate, infomercial review article?"
-
"Why did the Lancet, one the oldest and most respected medical journals on the planet, publish this industry-generated, inaccurate, infomercial review article?"
-
"Why would a Pharmaceutical Company want a favorable review article about their product published by a prominent doctor in a prestigious journal?"
The good news is that the right people were onto this article like flies onto honey as soon as it was published – responding in droves [see below]. Dr. Hickie should’ve known that the era of this kind of sheenanigans has finally passed and that his reputation will surely be forever tarnished by participating in this kind of ruse no matter how lofty his defense – because there is no defense. The other good news is that I understand that the Lancet editor has ‘abandoned this drug-focused type of review paper’ in the future altogether because of the bruhaha about this article.

Thanks for collating all of this in one place. Was very surprised to see a drug-focussed article that was not a systematic review (as stated on the front page) or a Cochrane review.
Without defending the authors or the journal, I suspect that the enormous pressure to publish on novel and (potentially) controversial topics were behind the poor decision-making on this one.
Do you have a source for the quote from Richard Horton in the last sentence?
Importantly Hickie’s BMRI organisation has put a lot of work into “chronobiology” and I suspect this study is partly the product of their faith in that particular biological reductionist view of depression and its treatment. They are also great fans of the Insel-ism “translational research”, only this time the research has been lost in translation.
This is a fool’s game. Dr. Robert Howland actually keeps hiding (past but recent) conflicts of interest (for example here: http://www.slackjournals.com/article.aspx?rid=88610), notably with Forest. No surprise then, that he gave such a good opinion on Viibrid: http://www.ncbi.nlm.nih.gov/pubmed/21323263.