There has been no recent issue as hotly contested as the question of whether the SSRIs can induce suicidal thinking, particularly in youth. In 2004, the FDA added a black-box warning to the SSRIs, resulting in a fall in prescribing, and a polarized literature on this topic that lives on. Recently, the focus has been on the Treatment for Adolescents with Depression Study [TADS], first published in 2004. I walked in on an email discussion of several recent articles and found myself kind of confused. So first, I thought I’d gather the relevant links for anyone who might want to read along [most articles available on-line]:
-
Treatment for Adolescents With Depression Study (TADS)
NCT00006286 on ClinicalTrials.gov -
Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents With Depression
by the Treatment for Adolescents With Depression Study (TADS) Team
JAMA. 2004 292(7):807-820. [full text on-line] -
Treatment for Adolescents with Depression Study (TADS): SafetyResults.
by Emslie G, Kratochvil C, Vitiello B, Silva S, et al.
Journal of the Amer Acad of Child and Adolesc Psychiatry. 2006 45(12):1440-55. -
The Treatment for Adolescents With Depression Study (TADS)
Long-term Effectiveness and Safety Outcomes
by The TADS Team
Archives of General Psychiatry. 2007 64(10):1132-1143. [full text on-line] -
Suicidal Events in the Treatment for Adolescents with Depression Study (TADS)
by Benedetto Vitiello, Susan Silva, Paul Rohde, Christopher Kratochvil, et al.
Journal of Clinical Psychiatry. 2009 70(5): 741–747. [full text on-line]
-
Suicidal Thoughts and Behavior With Antidepressant Treatment
Reanalysis of the Randomized Placebo-Controlled Studies of Fluoxetine and Venlafaxine
by Robert D. Gibbons, Hendricks Brown, Kwan Hur, John M. Davis, and J. John Mann
Archives of General Psychiatry. Online February 6, 2012. [full text on-line]
Most suicidal events occurred in the context of persistent depression and insufficient improvement, without evidence of medication-induced behavioral activation as a precursor. Severity of self-rated suicidal ideation and depressive symptoms predicted emergence of suicidality during treatment. Risk for suicidal events did not decrease after the first month of treatment, suggesting the need for careful clinical monitoring for several months after starting treatment.
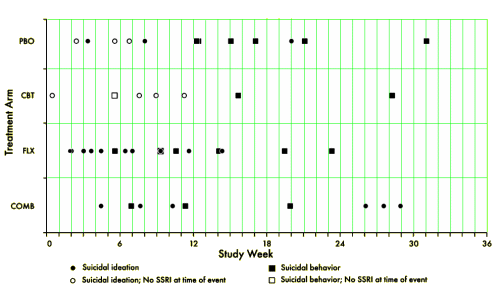
[reformatted for clarity]
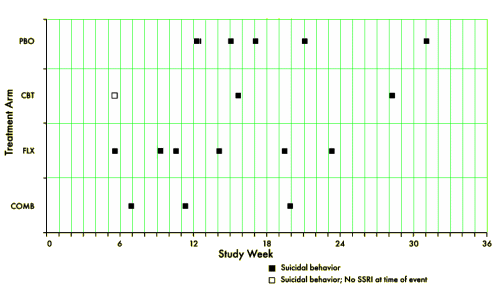
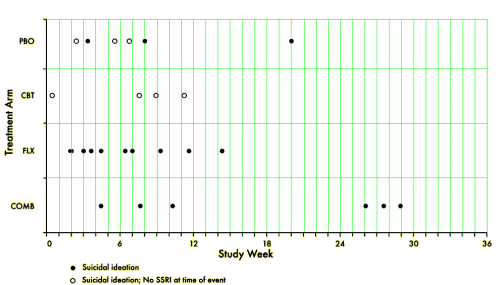
[reformatted to fit]
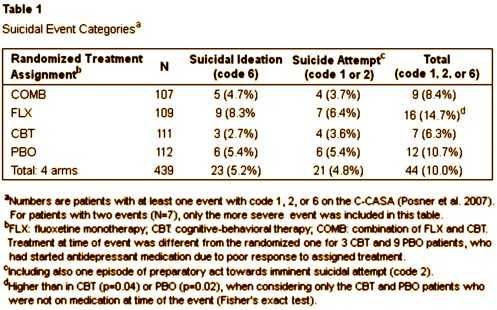
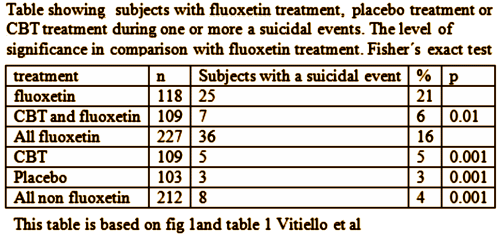
TADS was not a 36 week RCT, it was a 12 week RCT. In this article they claim in the abstract that it was a 36 week RCT. By doing that they can miscalculate the percentage with suicidal events. Suicidal events were defined as ideation or sucidal behaviour = added together as suicidal events. In the study after 12 weeks subjects were re-assigned, non-responding CBT and placebo participants were offered fluoxetine. We can clearly see the number of suicidal events – the black boxes and circles in figure 1. But they are not correctly assigned. If we are to give a percentage of subjects on placebo, all the subjects first on placebo and then on fluoxetine should be added to the fluoxetine group, leaving only true placebo. All the subjects who first had CBT and then got fluoxetine should be on the group of combined treatment. Both fig 1 and table 1 thus give false reporting, as percentage is calculated on the grouping of the first 12 weeks, not the full 36 weeks. The percentage of suicidal events in subjects on only fluoxetine, all fluoxetine, only CBT and combined CBT/fluoxetine is shown in my table.
What we have now is psychiatry researchers, having built their careers and, in some cases, fortunes, on flawed research enthusiastically recommending various psychiatric drugs, are in a rear-guard action to defend their legacies.
Do they even know they’re jimmying the data? Maybe not, their anosognosia being so acute they can’t even do math anymore, or have lost the ability to distinguish categories.
a·no·sog·no·si·a -n
-n
 s
s g-n
g-n
 z
z –
– , -zh
, -zh
noun
Unawareness of one’s disease, disability, or a defect.
My new favorite word!
You’ll love this NY Times article series by Errol Morris:
The Anosognosic’s Dilemma: Something’s Wrong but You’ll Never Know What It Is
http://opinionator.blogs.nytimes.com/2010/06/20/the-anosognosics-dilemma-1/
Your term ‘massaged’ and Whitaker’s term ‘hiding in plain sight’ are to the point here. The authors of the Vitiello paper have plausible deniability – after all they did report the data in the Figure. But they make the reader work to see the data clearly. That’s usually called obfuscation. Notice, for instance, how they do not forthrightly label the Figure as ‘suicide attempts on fluoxetine’ and ‘suicide attempts not on fluoxetine.’ Plus, fluoxetine in the Figure legend becomes the pastel, generic term SSRI. Did they learn this stuff from Madison Avenue?
The numbers speak for themselves: the Odds Ratio for suicide attempt being associated with current fluoxetine intake rather than no drug is 17. The rate ratio is 15 (7.5% versus 0.5%). The probability is 0.0005. Suppose the data had come out showing the reverse, that the drug has a huge preventive action against suicide attempts – do you think that finding would have been front and center in the publication? Duh.
There may be clinical reasons for this strong association of suicide attempt with on-drug condition in the TADS project. For instance, quite a few attempts occurred in patients who were switched to drug because of non-improvement with their earlier treatment. So the on-drug cases from week 12 to week 36 may have been more at risk. But looking into that is a second stage of discussion… first, the big effect has to be acknowledged, but it was obfuscated by Vitiello and co-authors.
As an aside, I am constantly amused to observe that physicians have no problem with the idea that some drugs that have a generally beneficial action on the brain can have unpredictable side effects such as the motor dyscontrol (dyskinesias) caused by l-DOPA in Parkinson disease, but the same people go postal when it is suggested that something similar might happen in the form of emotional and behavioral dyscontrol (agitation, akathisia, and suicide attempts) in some patients with SSRI drugs.
The connection of no meds ever vs on Zoloft was suicidal reaction in plain sight, when it happened to my child in 2001. All of the doctors saw it. Too bad their KOLs and data was based on lies and deceipt, otherwise they may have known not to give a teen (13!) Zoloft, or even though history was charted, the PROZAC at age 17. Always the same reaction, and thankfully my child did not kill herself on the drugs, just hanging out a car door on the freeway and other dreadful drug-induced, never repeated off the drugs events and behaviors.
I wrote my own abstract in 2001 showing the connection of SSRI use and increased suicidality, it’s in my boxes with the other momentos of those horrific days where doctors said they knew what they were doing.
Thank you, quacks!