| Editorial Coment |
| I’m showing these studies to look at the antidepressant-suicidality-in-adolescents question, but before getting to that, there’s something else. I was taught to be vigilant for graphmanship [using tricks to make graphs look better], so I usually replot data full-scale as I’ve done here. My comment is "no great shakes" in any of these studies. The major significant is time, not treatment effect [if penicillin worked like this, it would’ve probably never been discovered]. So treatment effects here may be significant, but they’re hardly exciting. |
The question of the use of antidepressants in depressed children and adolescents lives on [and on]. Previously, I’ve reported the industry funded studies shown here with the drug/date [see tuning the quartet…]:
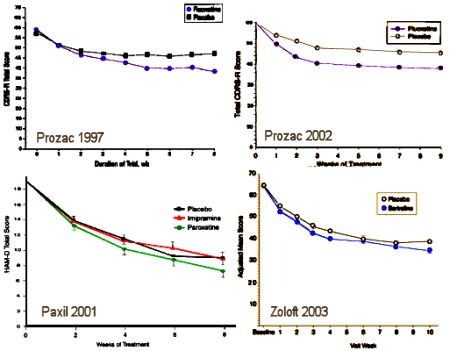
[replotted to full scale]
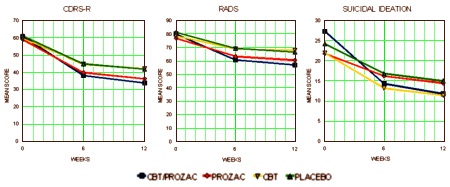
Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents With Depression
Treatment for Adolescents With Depression Study (TADS) Randomized Controlled Trial
Journal of the American Medical Association. 2004 292:807-820.
[full text on-line]
Objective: To evaluate the effectiveness of 4 treatments among adolescents with major depressive disorder.
Design, Setting, and Participants: Randomized controlled trial of a volunteer sample of 439 patients between the ages of 12 to 17 years with a primary Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnosis of major depressive disorder. The trial was conducted at 13 US academic and community clinics between spring 2000 and summer 2003.
Interventions: Twelve weeks of (1) fluoxetine alone (10 to 40 mg/d), (2) CBT alone, (3) CBT with fluoxetine (10 to 40 mg/d), or (4) placebo (equivalent to 10 to 40 mg/d). Placebo and fluoxetine alone were administered double-blind; CBT alone and CBT with fluoxetine were administered unblinded.
Main Outcome Measures: Children’s Depression Rating Scale-Revised total score and, for responder analysis, a (dichotomized) Clinical Global Impressions improvement score.
Results: Compared with placebo, the combination of fluoxetine with CBT was statistically significant (P=.001) on the Children’s Depression Rating Scale-Revised. Compared with fluoxetine alone (P=.02) and CBT alone (P=.01), treatment of fluoxetine with CBT was superior. Fluoxetine alone is a superior treatment to CBT alone (P=.01). Rates of response for fluoxetine with CBT were 71.0% (95% confidence interval [CI], 62%-80%); fluoxetine alone, 60.6% (95% CI, 51%-70%); CBT alone, 43.2% (95% CI, 34%-52%); and placebo, 34.8% (95% CI, 26%-44%). On the Clinical Global Impressions improvement responder analysis, the 2 fluoxetine-containing conditions were statistically superior to CBT and to placebo. Clinically significant suicidal thinking, which was present in 29% of the sample at baseline, improved significantly in all 4 treatment groups. Fluoxetine with CBT showed the greatest reduction (P=.02). Seven (1.6%) of 439 patients attempted suicide; there were no completed suicides.
Conclusion: The combination of fluoxetine with CBT offered the most favorable tradeoff between benefit and risk for adolescents with major depressive disorder.
[replotted to full scale]
[reformatted and abbreviated]

…and we’ve spoken of little else since that time, even though these drugs were never approved for pediatric use by the FDA. Their Warning was obviously heeded:
Suicidal Thoughts and Behavior With Antidepressant Treatment
Reanalysis of the Randomized Placebo-Controlled Studies of Fluoxetine and Venlafaxine
by Robert D. Gibbons, PhD; C. Hendricks Brown, PhD; Kwan Hur, PhD; John M. Davis, MD; and J. John Mann, MD
Archives of General Psychiatry. Published online February 6, 2012.
Context: The US Food and Drug Administration issued a black box warning for antidepressants and suicidal thoughts and behavior in children and young adults.
Objective: To determine the short-term safety of antidepressants by standard assessments of suicidal thoughts and behavior in youth, adult, and geriatric populations and the mediating effect of changes in depressive symptoms.
Data Sources: All intent-to-treat person-level longitudinal data of major depressive disorder from 12 adult, 4 geriatric, and 4 youth randomized controlled trials of fluoxetine hydrochloride and 21 adult trials of venlafaxine hydrochloride.
Study Selection: All sponsor-conducted randomized controlled trials of fluoxetine and venlafaxine.
Data Extraction: The suicide items from the Children’s Depression Rating Scale–Revised and the Hamilton Depression Rating Scale as well as adverse event reports of suicide attempts and suicide during active treatment were analyzed in 9185 patients (fluoxetine: 2635 adults, 960 geriatric patients, 708 youths; venlafaxine: 2421 adults with immediate-release venlafaxine and 2461 adults with extended-release venlafaxine) for a total of 53 260 person-week observations.
Data Synthesis: Suicidal thoughts and behavior decreased over time for adult and geriatric patients randomized to fluoxetine or venlafaxine compared with placebo, but no differences were found for youths. In adults, reduction in suicide ideation and attempts occurred through a reduction in depressive symptoms. In all age groups, severity of depression improved with medication and was significantly related to suicide ideation or behavior.
Conclusions: Fluoxetine and venlafaxine decreased suicidal thoughts and behavior for adult and geriatric patients. This protective effect is mediated by decreases in depressive symptoms with treatment. For youths, no significant effects of treatment on suicidal thoughts and behavior were found, although depression responded to treatment. No evidence of increased suicide risk was observed in youths receiving active medication. To our knowledge, this is the first research synthesis of suicidal thoughts and behavior in depressed patients treated with antidepressants that examined the mediating role of depressive symptoms using complete longitudinal personlevel data from a large set of published and unpublished studies.
| Another Editorial Coment |
| I’ll bet you think I put all this data in this post because I was going to try to resolve this quagmire of conflicting opinions and points of view. That would be a reasonable thing to conclude, but there’s an extenuating circumstance. I really put it here for you to read what the experts think and see what you think. I’m not an expert, but I have a likely immutable position on this topic, one that was reinforced by Dr. Healy [not the blog post referenced above, but by his recent book Pharmageddon]. See, I saw a case, and one was enough… |
I mentioned this case before but I’m going to tell the story again [pretty loud coi…]. I’m an adult psychiatrist and was not much of a prescriber of medications before I retired [2003]. I had two cases before then where shortly after starting Prozac, the patients returned saying they had severe anxiety and felt "crazy" – stopping it immediately. I don’t recall the year, but after the second one, I stopped using it altogether in the cases that came along where an SSRI seemed called for. There were plenty of other choices. I knew about the withdrawal syndromes of Paxil and Effexor, so I didn’t use them either. After I retired, I stayed away from doctoring for a while, then a few of years ago, I started volunteering at several charity clinics up here in the mountains – seeing adults, kids, and adolescents – since I’m the only act in town, in the county. Since it was clinic practice, I knew I’d have to brush up on psychopharmacology so I bought a few books and got on the Internet. I still work in the clinics, but "brushing up" turned into blogging about this stuff because I didn’t at all like what I found out.
The case at hand was a 16 or 17 year old boy who was plenty depressed. He was starting his last year of high school. It didn’t take very long to figure out why he was so down. His mother had remarried several years before, a retired Army Sergeant who wasn’t "over it." He and the boy had butted heads from the start and there were a jillion rules – unreasonable rules. By the time I saw him, the boy was clinically depressed by anyone’s criteria. He [and his mother] saw the only solution being his leaving home, and the great hope was getting into a diesel mechanic school in Tennessee after graduating. As time for his interview at the school approached, he seemed almost too depressed to even go, so I decided to see if an SSRI might give him some symptom relief and get him through it.
That was a bad decision as it turned out. By the second day on the medication, he was agitated, thinking "crazy," acting strange, filled with aggressive hostile thoughts, not sleeping. His mom called and we stopped the medicine post haste. Within a day and a half, the symptoms cleared. When we talked later, it was clear to all three of us that what happened had nothing to do with his depression or situation, that it was something else – an adverse reaction to the drug. I did some more "brushing up" and it was obviously the kind of reaction that warning up there is about. People call this anecdotal evidence – and these days, it doesn’t carry the weight of all those studies mentioned earlier, but before I get to that, let me finish the case. It has a decent ending. This mother realized the same thing I did. I had put him on medicine to try to help him with something and drug reaction aside, it was an error because that’s not what was wrong. So she abruptly left her husband shortly thereafter, although it left her destitute. Some time later, she came in and told me what she’d done and described her husband and her marriage in greater detail, leaving little wonder why the boy was so depressed [she was too]. She found a job, then a better one, and is now okay. The boy is a diesel mechanic trainee, glad of it, and doing fine. As for me, I learned something [and felt very lucky I didn’t do more damage].
This post is about Dr. Healy’s book Pharmageddon. It’s the book review I said I couldn’t muster earlier. I had to let it sit a while until it came together with my experience over the last almost half century. His book is about the forces that moved medicine from its traditional values, where every anecdote mattered and was evaluated using the tools of the scientific method – where every case was approached individually, not as a member of a class or a DRG [Diagnosis Related Group] to treat with some class-specific algorithm. If that sounds too lofty or nostalgic, it won’t if you think of yourself as the case, or when you are a case, or after you read the book. He shows how the concepts of evidence-based medicine, statistics, and the use of clinical trials came into being for decent reasons, but how they’ve become perverted by the Manage Care industry aiming to save money and the Pharmaceutical industry aiming to make money. He weaves a complex tale about how doctors have moved from treating diseased people more and more into treating ‘lifestyle’ – psychoactive drugs, statins, phosphates, generic treatments of classes – salesmen for a hungry industrial complex. Anecdotes are out, group-think is in. And he shows that it’s Medicine that has changed, not just Psychiatry – and not for the better. His predictions are dire, but his information and his logic are solid. It’s a broad hypothesis, too intuitively sound to discount. By the way, did I recommend you read it?
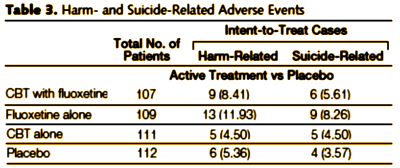
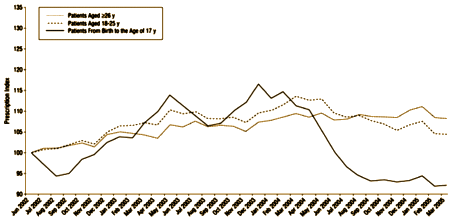
The problem that I’ve seen over and over from personal experience and from anecdotal reports from other patients is that when an adverse reaction is described as rare, doctors think it can’t show up in their practices. Even when it’s a bald example of an FDA black-box warning, they’ll ignore it or deny it.
This happened to me with oral terbinafine — I got (fortunately temporary) liver damage from it, as the black box warned — but my internist insisted up and down I had autoimmune hepatitis and would need a liver transplant in the foreseeable future. Great anxiety and expense ensued before a second opinion cleared the whole thing up.
With psychiatric drugs, even crystal-clear severe adverse reactions are ignored because 1) the patient, diagnosed with a psychiatric condition, is considered to be an unreliable source of information, 2) severe adverse reactions are rare, therefore what’s in front of your eyes couldn’t possibly be one of them, and 3) doctors are trained to believe they can counter unpleasant side effects of psychiatric drugs with other drugs.
Is this because of statistics? Do doctors believe an incidence of 1% is so tiny they’ll never see it? Or is it because they just don’t want to know from adverse effects?
Do they not realize that every hard-won FDA warning is because thousands of people have already been harmed?
Yes, there is definitely something going wrong with medicine here.