
B4Z-MC-LYAQ — J.V. Aranda [PI] 1/11/01-1/31/03
Lilly Research Laboratories Safety and Efficacy of Tomoxetine or Tomoxetine Plus Fluosetine in the Treatment of Mixed Attentional and Affective Disorders.
 Lest you think I’ve gone mad as a hatter, let me orient you to why I’m searching for Study LYAQ. Dr. Robert Gibbons et al have reported two online retrospective analyses in the Archives of General Psychiatry in which they assert that Prozac is not associated with suicidal thoughts in Children and Adolescents [Suicidal Thoughts and Behavior With Antidepressant Treatment] and that Prozac is effective in treating Child and Adolescent Depression, regardless of the severity [Benefits From Antidepressants]. Since these conclusions are counter to much of the current wisdom from the literature on these topics, I’m trying to vet how that conclusion was reached [and it’s making me a bit mad as a hatter]. In the last post, I ran down the studies he used for his analyses. Of the four he lists, the three I’m sure of are:
Lest you think I’ve gone mad as a hatter, let me orient you to why I’m searching for Study LYAQ. Dr. Robert Gibbons et al have reported two online retrospective analyses in the Archives of General Psychiatry in which they assert that Prozac is not associated with suicidal thoughts in Children and Adolescents [Suicidal Thoughts and Behavior With Antidepressant Treatment] and that Prozac is effective in treating Child and Adolescent Depression, regardless of the severity [Benefits From Antidepressants]. Since these conclusions are counter to much of the current wisdom from the literature on these topics, I’m trying to vet how that conclusion was reached [and it’s making me a bit mad as a hatter]. In the last post, I ran down the studies he used for his analyses. Of the four he lists, the three I’m sure of are:
| STUDY | DX | SPONSOR | YEAR | N | PBO | FLX | DURATION | 1BOM LINK |
|
|
||||||||
| X065 | MDD | NIMH? | 1991 | 96 | 48 | 48 | 6 weeks | {LINK} |
| HCJE | MDD | Lilly | 1998 | 219 | 110 | 109 | 6 weeks | {LINK} |
| TADS | MDD | NIMH | 2000 | 221 | 112 | 109 | 6 weeks | {LINK} |
The left-most column links to what’s available on the web from the study. The rightmost column links to my own previous reviews of the study.
I still can’t find Study LYAQ on clinical trials, pubmed, or Drugs@FDA under either Prozac or Strattera, but it was on the Lilly Clinical Trials site as a Strattera study. It wasn’t at all what I expected so I’m going to review what I did find there [I told you I might linger for a while on this article]. Here’s the description of the study:

Well Study LYAQ doesn’t look exactly like a clinical trial of Prozac in children and adolescents with Major Depressive Disorder to me. They obviously used the data from the Prozac-naive group. What were the criteria to get into the study?

Well the subjects were either sad or anxious ADHD kids or depressed kids with ADHD-ish symptoms. The real point of the study was to see if being on Prozac messed with the Strattera levels [it did – here]. The Primary Efficacy parameter was the CGI-S and it was not significant [p=0.065]. Here are the Secondary Outcomes:
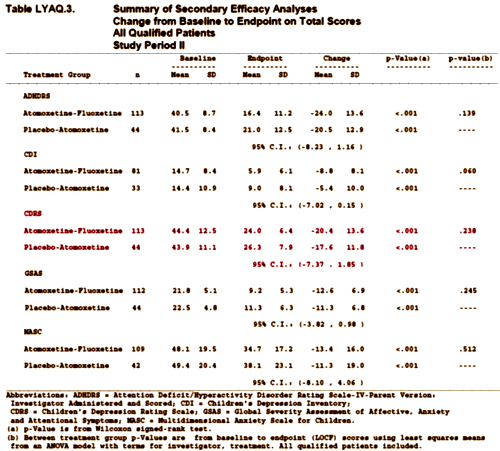
Note that the Children’s Depression Rating Scale [CDRS] is not significant between the two groups [Strattera+Prozac and Strattera+Placebo][p=0.238].
This is, of course, outrageous. Dr. Gibbons et al have included a study in their analysis where all of the subjects were also on a hefty dose of Strattera [Amoxetine] and the point of the study was to see what Prozac did to Stattera levels. The criteria for entering the study were anything but uniformly related to Major Depressive Disorder. The CDRS was a secondary efficacy variable and the study differences were not significant. And the mean CDRS scores were low [about the level where the studies of MDD end up after treatment (44.4 and 43.9)]! No one would buy that this study belongs in a serious study of the effectiveness of Prozac in treating depressed children if they knew where this study came from. Dr. Gibbons et al knew what this study was and didn’t tell us. The way we found it out was to spend a few days digging [being helped by other diggers]. In my book, this is an example of conscious and deliberate deceit.
But at least I can at last say that this is where their study data came from:
| STUDY | DX | SPONSOR | YEAR | N | PBO | FLX |
|
|
||||||
| X065 | MDD | NIMH? | 1991 | 96 | 48 | 48 |
| HCJE | MDD | Lilly | 1998 | 219 | 110 | 109 |
| TADS | MDD | NIMH | 2000 | 221 | 112 | 109 |
| LYAQ | MDD or ADHD or Anxiety in Combo |
Lilly | 2001 | 172 | 45 | 127 |
Thanks Mickey.
This is riveting blogging and sleuthing. Thank you for your hard work.