First off, I realize I’m stuck in a loop. I can’t seem to get away from the European Medicines Agency’s U Turn on Data Transparency. So I guess I’ll be stuck here until I’m not – because that didn’t just happen – it was orchestrated and we know who did it and a bit about how. In some further information… we were told by Peter Doshi and the BMJ about the bargaining by AbbVie and InterMune. In in the shadows… we saw how the PhRMA and EFPIA schemed in a concerted attack on Data Transparency aimed at the EMA. And now from from Trudo Lemmens, we’re shown a letter from PhRMA to the Chair of the US Trade Policy Committee lobbying the US to support their position. I pulled a few representative paragraphs:
 May 10, 2013 Mr. Douglas Bell
Chair, Trade Policy Staff Committee
Executive Office of the President
600 17 th Street, N.W.
Washington, D.C. 20508
Re: Request for Comments Concerning the Proposed Transatlantic Trade and Investment Partnership, 78 Fed. Reg. 19566 [Apr. 1, 2013] [page 2] Another issue of concern to the industry is the EMA’s current and proposed data disclosure policies. The biopharmaceutical industry is firmly committed to enhancing the public health through responsible reporting and publication of clinical research and safety information. Companies publish their research, collaborate with academic researchers, and disclose clinical trial information at the time of patient registration, drug approval, and for medicines whose research programs have been discontinued. However, disclosure of companies’ non-public data submitted in clinical and pre-clinical dossiers and patient-level data sets risks damaging public health and patient welfare. PhRMA and its members urge the U.S. government to engage with the EU in every available venue to ensure responsible data sharing that protects patient privacy, maintains the integrity of the regulatory review process, and preserves incentives for biomedical research by adequately shielding confidential commercial information from inappropriate disclosure. The EMA’s current and proposed data disclosure policies jeopardize these principles… [page 12] The EMA’s current practices, in addition to its proposed policies to proactively disclose companies’ non-public data submitted in clinical and pre-clinical dossiers and patient-level data sets, risk damaging public health and patient welfare. Government disclosure of companies’ unprocessed, non-contextualized raw data and technical analysis provides little benefit to practicing healthcare professionals and their patients. On the contrary, disclosure of such clinical trial data, including confidential commercial information, threatens patient privacy by facilitating patient re-identification from anonymized patient-level data sets; encourages second guessing of the EMA’s expert regulatory decisions, thereby undermining patient trust in the safety and effectiveness of approved medicines; and harms incentives to invest in biomedical research. The primary beneficiaries of such non-public information are competitors who wish to free-ride off of the investments of the innovators. Further, failing to protect confidential commercial information contained in regulatory submissions is inconsistent with the EU’s treaty obligations contained in the Agreement on Trade-Related Aspects of Intellectual Property Rights [TRIPS]. In order to benefit public health in the long run, data disclosure policies must preserve patient privacy, respect the integrity of regulatory systems, and maintain incentives to invest in innovative medical research, consistent with 21 C.F.R. §§ 312.130; 312.45(c); 314.430; 601.51(c) and Article 39(3) of TRIPS. For these reasons, PhRMA and its members urge the U.S. Government to engage with the EU in every available venue to resolve this issue…
|
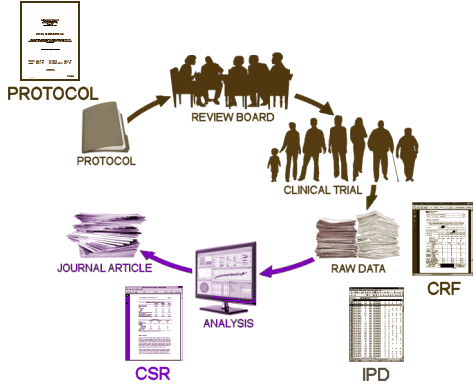
I formatted this for the blog last night, and highlighted parts of it. When I got back to it after work today, I found myself highlighting the rest of it. So I just gave up and let it stand as it is. It’s all infuriating. Data Transparency doesn’t compromise subject’s privacy. It doesn’t reveal confidential commercial information. In fact, this whole business about CSR‘s is a ruse. We don’t care about their carefully crafted CSR narratives. All we really want is the a priori protocol; the IPDs for the numeric and categorical results; and access to the CRFs to check the IPDs and look at the raw comments on AEs. It’s no harder to spin a CSR than a published paper [if these acronyms put you off, see in the details…, achilles’ heel…, and the wrong compromise… and this figure]:
I appreciate you keeping up with this subject and summarizing it for your readers. I have neither the time or the energy to expend on this so having a concise description to refer to helps me understand the history around the subject.
For the use of Article 39, I understand it completely. It may have even been written with the intent of allowing what has happened with drug trials (and other business malpractices) to occur. Although it wasn’t a pharmaceutical company, I have worked in the belly of the beast in a different industry, and this is not surprising to me at all.
Dr. Goldacre is not the only naive person and the comments that I keep leaving here about the way business operates are not intended to be rants by a bitter person lashing out. They were and are always written with the intent to call attention to what you are really fighting. And, what that is transcends any political party or any socioeconomic system. The ones calling the shots here are blood-thirsty, viscous, conniving and deceitful. They care no more for a company than they do for their customers, employees, shareholders, their own country or the health of mankind. That they get so many to follow them is based on a tease that a follower can become one of them. Of course some do, that is why they keep growing them, but the majority can never be one of them and they are just useful idiots to them. And those that don’t want to play this game end up as collateral damage. This is not just businessmen as the people in government (in both parties) that assist them are just like them. Dr. Hassman calls them sociopaths and I have to say I agree with his assessment.
I do hope we stop fighting amongst ourselves as Altostrata wrote, and fight them for the truth in many areas. Yet don’t be deceived, even if you win a small victory don’t ever think you’ve beaten them. They never truly lose. Even if it were possible to legally destroy every large pharmaceutical company, those in charge would still walk away with millions and move on to a different industry to wreak havoc there.
I don’t mean to sound so dark. On the contrary, I am a fairly easy going person that loves animals, sunsets, rainbows, fireworks, sleep, and simple pleasures like ice cream sandwiches, Tootsie Roll Pops and colors of nature in the Fall. Which is probably why I ended up as collateral damage in Corp. America.
Arby. To my thinking you’ve won. It’s painful to look the ugly truth squarly in the eye.
Anyone and everyone may end up as collateral damage, trusting a doctor and taking the legally marketed and prescribed medication.
Anyone with a concience and integrity, trying to work within the system, must become collateral damage or lose oneself.
Outsiders are less vulnerable. Those of us who know we have been betrayed, who lost loved ones, are least likely to become collateral damage, I think.
Thank you again, dr Mickey, for your exceedingly valuable work.
I love animals, etc., too. And I am collateral damage from bad clinical information about psychiatric drugs. I trusted my doctors and my life was destroyed. I am sitting among the ruins now. I was 54 when I was injured, I am 64 now. I am an old woman. I will never get those years back.
Thanks, berit.
Still, I needn’t have won because it isn’t about me. I only want to see the truth known. That sounds clique, but it is the way I roll.
Sometimes I answer questions that weren’t asked here, and what I answered above was the constant dismissal of my concerns as just me being negative. I’ve seen mouths hang open when I have been happy with an outcome at work because it so surprised my managers; they much preferred to assign negativity to me than to deal with the issues I raised..
I am truly sorry for everyone that is collateral. damage. I’ve never referred to people outside any system as that, because I always considered them to be the damage. But, I see where I was wrong. Very few in the world deliberately seek to out to destroy others, but there are many that don’t care about the consequences of their actions. Maybe a more correct term for my situation would be a recipient of friendly fire?