 Irving Kirsch published an article in 2008 [Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration] that concluded:
Irving Kirsch published an article in 2008 [Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration] that concluded:Drug–placebo differences in antidepressant efficacy increase as a function of baseline severity, but are relatively small even for severely depressed patients. The relationship between initial severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among very severely depressed patients, rather than to increased responsiveness to medication.
This new article capitalizes on the 2004 court order that GSK must post all of its clinical trials on a publicly available web site [GSK Clinical Study Register]. The amount of information on each study is highly variable – from the complete CSR and IPD for Study 329 [the proband for the court order] to short summaries for many other Clinical Trials. But still, we know all of the trials, so the unpublished studies problem evaporates. While the variable amount of data limits what can be done, this article looks at every RCT done by GSK on Paroxetine that used the Hamilton Rating Scale for either Anxiety [HRSA] or Depression [HRSD], and limited its scope to efficacy [not adverse events]. The key parameter, Effect Size [Cohen’s d] was calculated from reported means, standard deviations, and number of subjects – not derived from full data sets. In spite of these limitations, the study seems well done and has plenty to say:
A Meta-Analysis of Change on the Hamilton Rating Scalesby Michael A. Sugarman, Amy M. Loree, Boris B. Baltes, Emily R. Grekin, and Irving KirschPLoS·ONE. 08/27/2014 DOI:10.1371/journal.pone.0106337
Background: Previous meta-analyses of published and unpublished trials indicate that antidepressants provide modest benefits compared to placebo in the treatment of depression; some have argued that these benefits are not clinically significant. However, these meta-analyses were based only on trials submitted for the initial FDA approval of the medication and were limited to those aimed at treating depression. Here, for the first time, we assess the efficacy of a selective serotonin reuptake inhibitor [SSRI] in the treatment of both anxiety and depression, using a complete data set of all published and unpublished trials sponsored by the manufacturer.Methods and Findings: GlaxoSmithKline has been required to post the results for all sponsored clinical trials online, providing an opportunity to assess the efficacy of an SSRI [paroxetine] with a complete data set of all trials conducted. We examined the data from all placebo-controlled, double-blind trials of paroxetine that included change scores on the Hamilton Rating Scale for Anxiety [HRSA] and/or the Hamilton Rating Scale for Depression [HRSD]. For the treatment of anxiety [k = 12], the efficacy difference between paroxetine and placebo was modest [d = 0.27], and independent of baseline severity of anxiety. Overall change in placebo-treated individuals replicated 79% of the magnitude of paroxetine response. Efficacy was superior for the treatment of panic disorder [d = 0.36] than for generalized anxiety disorder [d = 0.20]. Published trials showed significantly larger drug-placebo differences than unpublished trials [d’s = 0.32 and 0.17, respectively]. In depression trials [k = 27], the benefit of paroxetine over placebo was consistent with previous meta-analyses of antidepressant efficacy [d = 0.32].Conclusions: The available empirical evidence indicates that paroxetine provides only a modest advantage over placebo in treatment of anxiety and depression. Treatment implications are discussed.
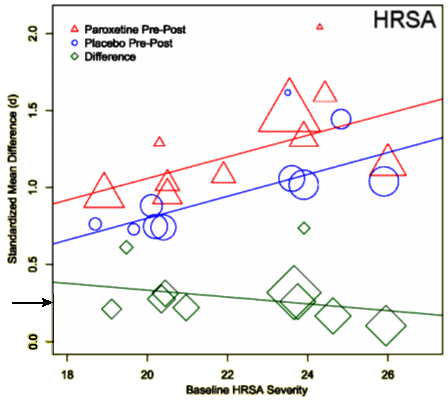
In this case, the red and blue lines showing the changes in drug and placebo over the course of the study and their significant change with severity are consistent with the regression to the mean error [your on your own her]. For my purposes, ignore them. The bottom green line shows the strength of effect for the drug. It is not significantly related to the severity of the Anxiety State. The mean Cohen’s d is 0.27 [I’ve marked it with a horizontal arrow] [recall that a rough interpretation of Cohen’s d is: 0.25 = weak effect, 0.50 = moderate effect, and 0.75 = strong effect]. From this graph, we can conclude that Paroxetine has a definite anxiolytic effect, but that it’s not anything to write home about. It’s not inert, but it’s nowhere even close to a wonder drug.
Now to the meta-analysis of the Paroxetine trials in depressed patients. The opposite effect of severity on the pre-post drug and placebo response [red and blue lines] was not clear to me, but unlike the meta-analysis in 2008 of FDA Approval studies, there was not a significant effect of severity on response. The mean Cohen’s d in depression is 0.32 [I’ve again marked it with a horizontal arrow].
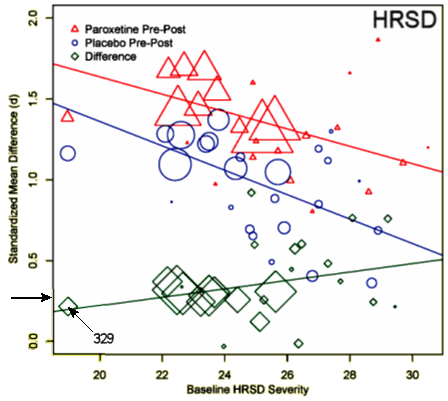
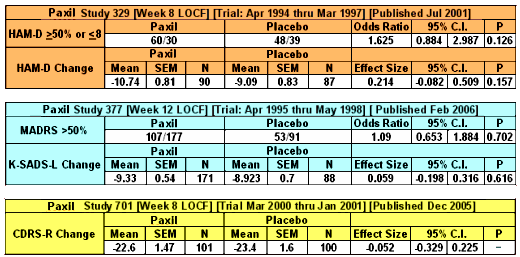
The only study in adolescents [the infamous Study 329] is also marked. The other GSK adolescent trials [published after the patent expired] used other rating systems [MADRS, K-SADS-L and CDRS-R] and were both decidedly negative [see paxil in adolescents: “five easy pieces”…].
They also assessed the impact of the trial length on the strength of the effect and found no significance:
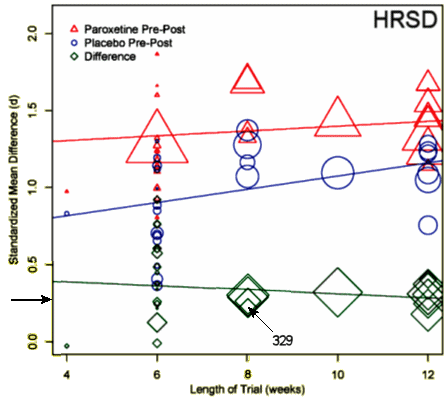
…the mean paroxetine-placebo effect size did not differ significantly as a function of approval status [Q[1] = 3.27, p = .077], although there was a trend towards a greater drug-placebo benefit in pre-approval trials [Pre-Approval: d = 0.41 [95% CI: 0.30,0.53]; Post-Approval: d = 0.29 [95% CI: 0.22,0.36]].
The weighted mean difference between paroxetine and placebo was not significantly different between published and unpublished trials [Q[1] = 1.50, p = .221]. Published trials [k = 16] had a weighted mean effect size of d = 0.36 [95% CI: 0.27,0.44] and unpublished trials [k = 11] had an effect size of d = 0.28 [95% CI: 0.20,0.37].
They didn’t look at the critical Adverse Event data, so this meta-analysis only addresses one side of the risk benefit equation. They have a nice discussion of the Adverse Events in the discussion, but it is not data-based from their own work. This article is what a little-bit of Data Transparency can tell us, but it’s just not enough. There’s the company machine operating between most of this data and the raw clinical trial. Considering where we’ve lived for decades, I think it’s a landmark article, but only a marker along the road to where we need to be.
This study still says nothing about whether blinds were maintained in the studies. That data is either non-existent or never made available. In the rare cases where antidepressants have been compared to active placebo, they were not superior. SSRIs are no more effective than any of those older antidepressants (given our current data), so it can be inferred that SSRIs would not be superior to an active placebo. This is the real reason you do not see SSRIs being compared to active placebo. We also know that in the rare cases where questions about placebo vs. drug were asked the blind is broken by 80-90% of patients and 90% of the clinician raters. These are not double-blind trials. There is no blind.
In his book Kirsch presents a lot of solid evidence that SSRIs have no treatment effect beyond placebo effects. I have seen no other evidence to the contrary since his book has come out.
Paroxetine is anti-cholinergic so, yeah, anxiolytic, I guess you could say.
This paper is but a penlight in the murk. The huge body of self-referential literature, seeded by pharma-authored studies, recycled into Cochrane reviews, extolling paroxetine’s efficacy are what doctors remember, not these late correctives.
It will take generations for the textbooks to be cleaned of the misinformation. For example, in a handbook for PCPs, Current Medical Diagnosis & Treatment, the chapter on Psychiatric Disorders, authored by Eisendrath and Lichtmacher of UCSF, cites the STAR*D study for treatment recommendations for adults and children and the CATIE study for antipsychotic prescribing — as though their conclusions were valid.
That is the mainstream of thinking in psychiatry, not having budged an inch in 10 years. Of course, writing well-informed textbooks for psychiatry might be difficult. What is to be said of the most effective antidepressant, for example?
@Ryan
I made that exact point, that patients’ ability to break the blind causes an exaggeration in the reported effect, in an article that was supposed to run in Psychology Today summer of 2000. It was supposed to be the feature article and I used a hypothetical situation to show how the fact that subjects break the blind reduces the claimed effectiveness of antidepressants to almost 0. I said “supposed to run” because the advertising department screamed the if the article were published then they wouldn’t be able to sell their Prozac and Paxil ads, and the magazine censored the article, chopping it to a one-page sidebar.
I have to thank the managing editor at the time for fighting hard enough to get the side-bar published. Otherwise it would have been cut all together.
As the saying goes “Those who have the gold make the rules”. It was a very disillusioning experience for me.