PharmalotBy Ed SilvermanSeptember 8, 2014As the pharmaceutical industry battles with regulators over the extent to which clinical trial information should be released, one drug maker has run afoul of U.K. industry rules for disclosure. The Association of British Pharmaceutical Industry has ruled that Servier breached several codes by failing to publish trial results for its Valdoxan antidepressant. Specifically, there were five trials involving U.K. patients between 2008 and 2011 in which Servier did not publish results promptly and three other trials in which results had not been disclosed.
As a result, the drug maker breached three codes – bringing discredit upon, and reducing confidence in, the pharmaceutical industry; failing to maintain high standards and failing to meet the required timeframe in which to disclose clinical trials, according to the ABPI. The trade group monitors a voluntary industry code and late last week publicized the completed findings. Disclosing trial results has been a contentious topic that has immersed drug makers, regulators and researchers following scandals over safety or effectiveness data that was not publicly shared. The debate has mostly centered on the extent to which proprietary trade information, as well as confidential patient data, may be released.
As we wrote previously, the issue has been particularly troublesome in Europe, where the European Medicines Agency has repeatedly delayed the release of a new policy amid criticism the agency backtracked on a previous commitment to new-found openness. Recently, the regulator indicated formal adoption would take place at a scheduled board meeting next month. The case against Servier emerged after the ABPI last fall published a survey suggesting there was greater industry transparency concerning clinical trial data. An unidentified member of the public, however, suspected otherwise and, after reviewing the results, reported possible violations to the industry trade group.
For its part, Servier “strongly refuted” that it discredited the industry or failed to maintain high standards, and acknowledged only a technical breach concerning a failure to meet the required timeframe in which to disclose trial results, according to the case report. We asked the drug maker if an appeal is planned and will update you accordingly. Meanwhile, the ABPI findings were publicized in the form of advertisements that are running in three publications that are widely read in the U.K. medical industry – BMJ, the Pharmaceutical Journal and The Nursing Standard.
by Ian B Hickie and Naomi L RogersLancet 2011 378: 621–631.
Major depression is one of the leading causes of premature death and disability. Although available drugs are effective, they also have substantial limitations. Recent advances in our understanding of the fundamental links between chronobiology and major mood disorders, as well as the development of new drugs that target the circadian system, have led to a renewed focus on this area. In this review, we summarise the associations between disrupted chronobiology and major depression and outline new antidepressant treatment strategies that target the circadian system. In particular, we highlight agomelatine, a melatonin-receptor agonist and selective serotonergic receptor subtype [ie, 5-HT2C] antagonist that has chronobiotic, antidepressant, and anxiolytic effects. In the short-term, agomelatine has similar antidepressant efficacy to venlafaxine, fluoxetine, and sertraline and, in the longer term, fewer patients on agomelatine relapse [23·9%] than do those receiving placebo (50·0%). Patients with depression treated with agomelatine report improved sleep quality and reduced waking after sleep onset. As agomelatine does not raise serotonin levels, it has less potential for the common gastrointestinal, sexual, or metabolic side-effects that characterise many other antidepressant compounds.
[2] The bias in this paper is very disturbing – it might be fine to argue your case in a Viewpoint or letter. But…
[3] …this paper purported to be an unbiased review of a new drug class. Peer review improved it, yet not enough.
[4] As troubling is the fact that one author took part in speaking engagements for the company making one of these drugs.
[5] It is this kind of complicity that damages any hopes of a positive partnership between medicine and industry.
by Markus Koesters, Giuseppe Guaiana, Andrea Cipriani, Thomas Becker, and Corrado BarbuiThe British Journal of Psychiatry. 2013 203:179-18.
Background: Agomelatine is a novel antidepressant drug with narrative, non-systematic reviews making claims of efficacy.Aims: The present study systematically reviewed published and unpublished evidence of the acute and long-term efficacy and acceptability of agomelatine compared with placebo in the treatment of major depression.Method: Randomised controlled trials comparing agomelatine with placebo in the treatment of unipolar major depression were systematically reviewed. Primary outcomes were (a) Hamilton Rating Scale for Depression (HRSD) score at the end of treatment (short-term studies) and (b) number of relapses (long-term studies).Results: Meta-analyses included 10 acute-phase and 3 relapse prevention studies. Seven of the included studies were unpublished. Acute treatment with agomelatine was associated with a statistically significant superiority over placebo of –1.51 HRSD points (99% CI –2.29 to –0.73, nine studies). Data extracted from three relapse prevention studies failed to show significant effects of agomelatine over placebo (relative risk 0.78, 99% CI 0.41–1.48). Secondary efficacy analyses showed a significant advantage of agomelatine over placebo in terms of response (with no effect for remission). None of the negative trials were published and conflicting results between published and unpublished studies were observed.Conclusions: We found evidence suggesting that a clinically important difference between agomelatine and placebo in patients with unipolar major depression is unlikely. There was evidence of substantial publication bias.
Selective reporting
Study protocols were not available for all the studies. Data from CL3-022; CL3-023; CL3-024 were limited, as we could not gain access to the full dataset because the studies were unpublished; attempts to obtain data from the pharmaceutical company manufacturing agomelatine [Servier] were unsuccessful. Loo 2002a, CL3-022, CL3-023 and CL3-024 were not registered, thus increasing risk of selective reporting…
-
Patient-level clinical trial data, study-level clinical trial data, full clinical study reports, and protocols from clinical trials in patients for medicines approved in the United States and European Union will be shared with qualified scientific and medical researchers upon request and subject to terms necessary to protect patient privacy and confidential commercial information. Researchers who obtain such clinical trial data will be encouraged to publish their findings.
-
Companies will work with regulators to provide a factual summary of clinical trial results to patients who participate in clinical trials.
-
The synopses of clinical study reports for clinical trials in patients submitted to the Food and Drug Administration, European Medicines Agency, or national authorities of EU member states will be made publicly available upon the approval of a new medicine or new indication.
-
Biopharmaceutical companies have also reaffirmed their commitment to publish clinical trial results regardless of the outcome. At a minimum, results from all phase 3 clinical trials and clinical trial results of significant medical importance should be submitted for publication.

click the figure for full view
It may not seem like much, a blog post in the WSJ, a pdf on a rarely visited web site, a cryptic ad in the BMJ, but it’s big to me because it’s the pharmaceutical industry censuring a drug company for not publishing negative clinical trial results, thereby falsifying their drug’s efficacy. And look at what it took: a brace of well respected watchdogs from the Cochrane Collaboration, Healthy Skepticism, Dr. Carroll; candid comments from the Lancet editor; an article in the British Journal of Psychiatry; a Cochrane Systematic Review; echos from Senator Grassley, Paul Thacker, David Healy’s work, Ben Goldacre’s books, AllTrials, Fiona Godlee, Peter Doshi, Tom Jefferson, the irreplacable Ed Silverman, and the list goes on and on – a huge effort by a growing throng of contributors including even an anonymous, contactable member of the public, all focused on getting that one simple ad published in a few medical journals.
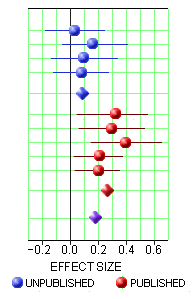
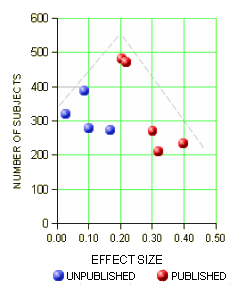
Thank you for this analysis, Dr. Mickey. I continue to be amazed by your speed and volume of output – after all, the Ed Silverman story just appeared yesterday.
Do we have examples of the US pharmaceutical industry doing this sort of thing? I think not.
And even ABPI/PMCPA couldn’t bring themselves to say what Servier did in respect of what drug. Lacking a bill of particulars, these published notices will be opaque to the average reader. But, as you say, it’s a start.