-
United States, California: For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon – Fri from 9 AM to 5 PM Eastern Time (UTC/GMT – 5 hours, EST), or speak with your personal physician. San Diego, California, United States, 92123
-
Russian Federation: For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon – Fri from 9 AM to 5 PM Eastern Time (UTC/GMT – 5 hours, EST), or speak with your personal physician. Lipetsk, Russian Federation, 399313
But where there’s a will, there’s a way. The ClinicalTrials.gov site includes a small link, History of Changes, with each trial up at the top of the opening page [go ahead, click it]. And when you get a pop-up window, there’s another link, ClinicalTrials.gov Archive Site [don’t be shy, click it too]. It has all the changes made over the course of the study. I’ve looked at a lot of these, but I’ve never seen one that looks remotely like this!
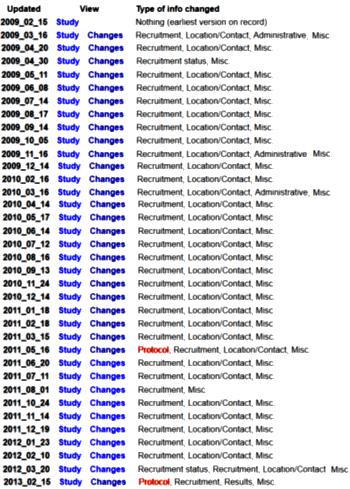
There are over thirty changes that have to do with recruitment and site location. If you have the energy, click on the second one [2009_03_16], then uncheck the box that says "Hide non-essential portions (contact info, locations, etc.)". You can scroll through the changes by clicking the arrow on the right. What you’ll see is them moving all over the world adding and shutting down different sites. The CRO must’ve had a hell of a time with recruitment and had to pull out all stops to find subjects. I want to know if that happened [and it ought to be easier to do]. It makes me think that the Pediatric Bipolar patients with a Depressive episode [or whatever these cases are] just aren’t that easy to find – but who knows?
If you’ve made it this far, you probably are wondering why I’m being so picky. Remember back in November, NIH Director Francis Collins announced that he was going to put teeth into the ClinicalTrials.gov website [Honoring Our Promise: Clinical Trial Data Sharing and see promises, promises…]. For one thing, the pharmaceutical companies and even academic researchers had just ignored the Results Section of ClinicalTrials.gov – a requirement for a lot of these trials [see transparency…]. They just didn’t ever get around to filing the results. And while the Results Section is pretty skimpy, it’s a right move in the right direction for DATA TRANSPARENCY. And part of that move was to enforce and even expand the Results Section. He put out a call for comments on his proposed plan, so I’ve been waiting for an industry RCT to come along so my comment will have example to show the why of my complaints – and this study is the very thing I’ve been waiting for. So the last blog, this one, and the nextl are my formulating what I want to say. Hope springs eternal in the new year. [This is the working link for comments: http://www.regulations.gov/#!documentDetail;D=NIH-2011-0003-0003 with a deadline of February 19, 2015].
FYI cost at lowest that I could find. For lowest dose
Fluoxatine 1 per day, for 30 days–$7.50
Olanzepine 1 per day for 30 days- $30
Symbyax 1 per day for 30 days $170. Most much higher.